Chemical Reaction of the Day
Short blocks of chemistry facts
Wednesday, August 28, 2013
The Compound Syria Supposedly Used as Chemical Warfare
There are a number of different gasses that have been used in chemical warfare, but the most likely culprit in the case of the recent Syrian civil war conflict is Sarin. Sarin is a potent nerve agent (a phosphorus containing molecule that inhibits nerves from communicating to organs), and is fairly easy to synthesize as it only requires two reactants, one of which is isopropyl alcohol, and the other methylphosphonyl difluoride. These reactants are combined when the nerve gas is released since this molecule is unstable in the P-F bond. The toxicity of this compound reaches about 500 times that of cyanide. The molecule was originally synthesized in 1938 by a German company in sight to produce an insecticide, but proved to be more useful in chemical warfare after the German army began testing in 1939.
What makes the use of chemical warfare, subject to such serious allegations? They are weapons of mass destruction that are cheaper, less destructive on the environment, and selective on anyone without a gas mask. So a regime, such as the Syrian army, can use it to eliminate it's citizens, while their army wears protective gas masks. And, as a nuclear blast would demolish a city, a chemical bomb leaves the buildings intact, but with a high death toll. These weapons aren't used to fight a war, but to exterminate an entire population within the desired vicinity. This is why chemical warfare has been banned.
Labels:
chemistry,
current events,
gas,
nerve gas,
news,
phosphorus,
poison,
war,
warfare
Thursday, August 15, 2013
Red Hippopotamus Sweat
Hippos have developed a very interesting form of sweat that possesses additional functionality than cooling down the body temperature. Before studied, the sweat was thought to be blood due to the red colour, but it was found to just be two pigmented molecules which have two functions for the hippo, being a sunscreen, and an antibiotic. It has been found that the main components aren't secreted as most sweat components are, but secreted in subdermal glands, making the classification of the sweat false as sweat is secreted from sweat glands, located in the epidermis.
The two molecules that are secreted are Hipposudoric acid and Norhipposudoric acid (seen below)
These molecules are conjugated systems, meaning in layman's terms that the bonds alternate single to double, but this also means that light is absorbed into this system ranging from the 200nm - 600nm wavelength. This property is what gives the sweat the sunscreen-like properties and the red colour where the Hipposudoric acid is red and the Norhipposudoric acid is orange. As for the antibiotic properties, this is the job of Hipposudoric acid, which inhibits two species of pathogenic bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae.
In finding this, there is an obvious potential for this to be used in consumer sunscreens as there is such a demand for natural sources in cosmetics. Now it's not like there would be a farm somewhere collecting hippo sweat, as there is a method for synthesizing Hipposudoric acid, published in 2006 in Tetrahedron Letters.
Reference
The two molecules that are secreted are Hipposudoric acid and Norhipposudoric acid (seen below)
Hipposudoric acid
Norhipposudoric acid
These molecules are conjugated systems, meaning in layman's terms that the bonds alternate single to double, but this also means that light is absorbed into this system ranging from the 200nm - 600nm wavelength. This property is what gives the sweat the sunscreen-like properties and the red colour where the Hipposudoric acid is red and the Norhipposudoric acid is orange. As for the antibiotic properties, this is the job of Hipposudoric acid, which inhibits two species of pathogenic bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae.
In finding this, there is an obvious potential for this to be used in consumer sunscreens as there is such a demand for natural sources in cosmetics. Now it's not like there would be a farm somewhere collecting hippo sweat, as there is a method for synthesizing Hipposudoric acid, published in 2006 in Tetrahedron Letters.
Reference
Wednesday, August 14, 2013
Minimalist Periodic Table
On my daily roundabouts of the internet I came across this beautifully constructed periodic table of elements created by Alison Haigh. Now this will not be able to get you anywhere as there is no other information on here other than the number of electrons, and you can only obtain that number if you wish to count a bunch of dots. Aside from the practicality point, this is one beautiful work of art that captured my chemist heart. Head to her website to check out the rest of the pictures, or to purchase a print of her work.
Source
Sunday, July 28, 2013
Monocrotophos: A Deadly Insecticide
A recent incident in India has left 23 children dead as a result of cooking oil contaminated with monocrotophos. Being as this was a free meal by government, aimed to keep 120 million children well fed and nourished, this turns some heads.
This molecule has been used as an insecticide, but has since been banned in Canada and the US, along with a number of other countries. They have failed to keep a ban on such pesticides due to the issue of having to feed so many hungry mouths, and needing a high crop output. In the countries where it is still legally used as a pesticide it is required to dilute it to 16% by volume of water. But in this case there was no dilution, indicating that there was direct contact with the poison after the oil processing.
This then becomes a question of who is responsible for insuring this doesn't happen again? One option is to enforce the correct usage and safety for this material, but considering the large illiteracy of the population, and difficulty in setting up an enforcement task, this poses much difficulty.
My opinion in how to prevent something like this from happening again is to enforce limitations on the manufacturer of the insecticide, the ones who enticed the Indian government into lifting the ban with their low price point. To either enforce a ban on synthesizing the compound all together, or to impose a concentration restriction, making the manufacturer dilute before the product is sold
Wednesday, July 17, 2013
The Chemistry of Cement
Cement, the binder used to hold objects together, most commonly used in concrete and mortar. The chemistry of cement can be thought of as a deconstruction of a chemical, only to reconstruct the same chemical, but in a different shape. This molecule that we will be looking at is Calcium Carbonate, a component of seashells, and limestone. To start the process of creating cement, the CaCO3 must be broken down into Calcium oxide through heating it at 825°C, in a process called lime calcination, possessing the equation CaCO3 → CaO + CO2. This Calcium oxide exists in a light powdery form, capable of being transported easily to wherever needs to be cemented. Once the cement is ready to be made, the CaO is mixed with water to produce Calcium Hydroxide, Ca(OH)2, in the equation CaO + H2O → Ca(OH)2. This watery paste is ready to be placed into the mould to set. In the setting process, the Calcium Hydroxide returns to it's original state of Calcium Carbonate, through a slow process of combining with the carbon dioxide within the air in the chemical equation Ca(OH)2 + CO2 → CaCO3 + H2O.
Wednesday, July 10, 2013
Mushroom Coral as a Sunscreen
In an episode of Man vs. Wild that I was watching, a Mushroom Coral's mucus was used as a sunscreen, so me as a chemist interested in cosmetics, I wanted to know what photoprotecting chemicals were in this mucus. The coral Fungia fungites and a couple other species gained this ability to protect themselves because they would grow in shallow water of the Adaman Sea where a lot of UV radiation penetrates through the clear water. First looking at this ability one would expect the coral to synthesize these molecules, but it turns out that the culprits are tiny symbiotic dinoflagellates. In a paper from 1999 by Brown1 studying the makeup of the mucus required for photoprotection, they look at the concentration of xanthophylls in the mucus, the primary photoprotector. Their study looked at how the concentrations of xanthophylls varried throughout the day, and showed that it is best to harvest the mucus at noon when the xanthophylls are at their peak concentration.
So finally to the chemistry of all of this. The molecule shown above is called diatoxanthin, which is a type of xanthophylls, and this is the molecule mostly responsible for filtering out the harmful rays. So how does this happen? Well molecules and atoms absorb light one way or another through absorbing the photons, resulting in electron excitation, and in this case, the molecule absorbs in the UV portion of the electromagnetic spectrum. So what happens after the diatoxanthin molecules become excited? Well biology has found a solution for that, which has been named the Xanthophyll Cycle. It's job is to convert all the used up diatoxanthin back into diatoxanthin.
References:
1. Fitt, W. K., R. P. Dunne, S. W. Gibb, D. G. Cummings, I. Ambarsari, B. E. Brown, and M. E. Warner. "Diurnal Changes in Photochemical Efficiency and Xanthophyll Concentrations in Shallow Water Reef Corals : Evidence for Photoinhibition and Photoprotection." Coral Reefs (1999): 99-105.
Monday, July 8, 2013
4-Methylimidizole, a Pepsi Additive
Recently the news has been discussing Pepsi's additive 4-methylimidizole in the caramel colouring of their popular drinks, with the fact that the Centre for Environmental Health has deemed it unsafe. Now should you go into your fridge right now and toss all of your Pepsi into the garbage? No. Although a study that was performed in 2007 gave results showing that 4-methylimidizole has carcinogenic properties in mice and rats, keep in mind that this is at an extremely high dose (115mg/kg of body weight) and the dosage that a normal consumer will receive showed no effects.
So why is this molecule in Pepsi in the first place? It comes with the additive caramel colour, which is produced by a separate manufacturer and is listed as Caramel Colour on the ingredients list. This molecule is made as a product of the Maillard Reaction, the browning of food, and in this case being the reaction to produce the caramel colour. But the problem with their method in producing the caramel colour is that they use a method with a higher concentration of ammonia, which incidentally ends up producing more 4-methylimidizole than other methods. Since this has come out, the US caramel colour manufacturers have changed their recipe to adhere to the people's reaction, lowering the overall concentration of 4-methylimidizole. This has yet to change the formulation in other parts of the world though.
Labels:
carcinogen,
chemistry,
current events,
drink,
food,
food chemistry,
maillard,
news,
organic,
pepsi
Thursday, June 27, 2013
How the Internet is Changing Your Brain
I was introduced to this video on Academic Earth in relation to my post about where science education should go, and it goes without saying that they harmonize each other very well. Please watch this 2 minute video, and it will open your eyes to what our technology is doing to our brains.
Created by AcademicEarth.org
Created by AcademicEarth.org
Tuesday, June 11, 2013
Why do Onions Make You Cry?
Cutting onions is arguably the worst part of cooking, the burning irritation you get in your eye becomes unbearable so much as to take a break mid way through chopping. So why is this? This is all to do with mixing molecules in the onion cells that aren't supposed to be together, an enzyme named Allilinase and a molecule called Allilin. Think of this like the type of adhesive that has two parts that you need combine together to make the glue active. When we cut onion we start the chain of reactions allowing the Allilin to come in contact with Allilinase to turn the Allilin into Sulfenic Acid and this Sulfenic Acid turns into syn-Propanethial-S-oxide though the enzyme Lachrymatory Factor Synthase.
This syn-Propanethial-S-oxide is what everyone hates because this is the irritant that when combined with the moisture in your eyes gives you that pain and your body tells your tear ducts to try and wash it out.
References:
Something about Science
Why Does Garlic Sometimes Turn Blue?
I've noticed sometimes when I cook garlic in butter that the garlic will turn blue after a while, and I had to ask myself why this happens. After finding out that it is safe and poses no loss of taste, I sighed and continued reading. It seems to be due to the presence of sulfates within the garlic, a component of what gives it such a pungent smell. What happens is that the sulfates combine with the copper ions present in the butter, water, or cooking equipment to create something known as Copper (II) sulfate, or CuSO4. This new compound is what gives off that bright blue colour.
So you may ask now if there are any precautions to be taken to avoid receiving this colour. Luckily science has gained us much information about this process. The main component of this phenomena is an enzyme present within the garlic that catalyzes this reaction, and can be deactivated simply by heating at high temperatures, or by letting the garlic age.
Monday, June 3, 2013
Hydrangeas and their Variety of Colour
Hydrangeas (Hydrangea macrophylla) have been known to form in a range of colours, from red, to purple, to blue, with having nothing to do with the genetics of the plant. So, if this colour difference isn't due to the genes, it must be due to certain environmental factors, and as it turns out, it is all to do with the pH and other properties of the soil. The more acidic the soil, and the greater [Al3+] in the soil, the more blue the sepals of the hydrangea will become. So why is this? Let's start with looking at the pigments which govern the colour of the sepals. The major pigment found in Hydrangeas (along with a number of other plants) is called Myrtillin, also known as delphinidin 3-glucoside, which is part of a group of molecules called anthocyanin.
But odly, this molecule assumes a red colour in acidic conditions and blue in basic conditions1 (opposite to that of the soil conditions) and the pH within the sepals is usually slightly acidic, with little variation, so the blue colouring must have something to do with the Al3+. Through much research, Kondo et al. 2, came up with a model proposing that the aluminum acts as a bridge to coordinate the Myrtillin to other copigments pigments known as acylquinic acids, which produces this blue colour.
While this is how the blue colour is formed, it can be safely assumed that the red colour is merely caused by Myrtillin by itself without any co-pigments or metal chelation. A question which hasn't been answered yet is what the pH of the soil has to do with all of this. The low pH allows there to be more free aluminum ions within the soil, to allow there to be more [Al3+] taken up into the plant.
References:
Second figure from 1.
1. K. Yoshida, M. Mori and T. Kondo, Nat. Prod. Rep., 2009, 26, 884–915.
2. T. Kondo, Y. Toyama-Kato and K. Yoshida, Tetrahedron Lett., 2005, 46, 6645–6649.
But odly, this molecule assumes a red colour in acidic conditions and blue in basic conditions1 (opposite to that of the soil conditions) and the pH within the sepals is usually slightly acidic, with little variation, so the blue colouring must have something to do with the Al3+. Through much research, Kondo et al. 2, came up with a model proposing that the aluminum acts as a bridge to coordinate the Myrtillin to other copigments pigments known as acylquinic acids, which produces this blue colour.
While this is how the blue colour is formed, it can be safely assumed that the red colour is merely caused by Myrtillin by itself without any co-pigments or metal chelation. A question which hasn't been answered yet is what the pH of the soil has to do with all of this. The low pH allows there to be more free aluminum ions within the soil, to allow there to be more [Al3+] taken up into the plant.
References:
Second figure from 1.
1. K. Yoshida, M. Mori and T. Kondo, Nat. Prod. Rep., 2009, 26, 884–915.
2. T. Kondo, Y. Toyama-Kato and K. Yoshida, Tetrahedron Lett., 2005, 46, 6645–6649.
Wednesday, May 22, 2013
How Bioluminescence Works
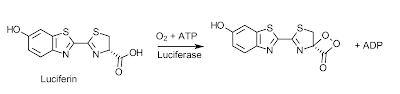
Bioluminescence is the ability of a living organism to emit light; a phenomenon which has intrigued humans for thousands of years and allowed them to invent mystical and magical stories behind these beacons of light. We might have decided that these lights serve a magical purpose, but evolution has found many uses for this process from protection, all the way to communication. The chemical mechanism behind these bioluminesent photons is a very simple one, though not the same in all organisms, they share some of the same basic principles. Taking the firefly as an example, the compound which acts as it's light bulb is called luciferin, and the reaction begins with molecular oxygen and ATP, with the help of the enzyme luciferase, turning luciferin into a dioxetane derivative (mechanism of this can be seen here)1.
Once this dioxetane derivative is formed the emission of light reaction is ready to occur spontaneously. This happens in a concerted reaction releasing CO2 and producing an excited ketone. The electron of the excited ketone then falls in energy to create a photon and end with the final product of oxyluciferin.
The newly created oxyluciferin is then enzymatically recycled into luficerin by this mechanism here2.
References:
1. Aldo Roda Chemiluminescence and Bioluminescence: Past, Present and Future, p. 57, Royal Society of Chemistry, 2010, ISBN 1-84755-812-7
2. Keiko Gomi and Naoki Kajiyama. Oxyluciferin, a Luminescence Product of Firefly Luciferase, Is Enzymatically Regenerated into Luciferin. J. Biol. Chem. 2001 276: 36508-36513. July 16, 2001, doi:10.1074/jbc.M105528200
The newly created oxyluciferin is then enzymatically recycled into luficerin by this mechanism here2.
References:
1. Aldo Roda Chemiluminescence and Bioluminescence: Past, Present and Future, p. 57, Royal Society of Chemistry, 2010, ISBN 1-84755-812-7
2. Keiko Gomi and Naoki Kajiyama. Oxyluciferin, a Luminescence Product of Firefly Luciferase, Is Enzymatically Regenerated into Luciferin. J. Biol. Chem. 2001 276: 36508-36513. July 16, 2001, doi:10.1074/jbc.M105528200
Labels:
atp,
biology,
bioluminescence,
enzyme,
excited ketone,
firefly,
hv,
ketone,
light,
organism,
peroxide
Subscribe to:
Comments (Atom)