Wednesday, August 28, 2013
The Compound Syria Supposedly Used as Chemical Warfare
There are a number of different gasses that have been used in chemical warfare, but the most likely culprit in the case of the recent Syrian civil war conflict is Sarin. Sarin is a potent nerve agent (a phosphorus containing molecule that inhibits nerves from communicating to organs), and is fairly easy to synthesize as it only requires two reactants, one of which is isopropyl alcohol, and the other methylphosphonyl difluoride. These reactants are combined when the nerve gas is released since this molecule is unstable in the P-F bond. The toxicity of this compound reaches about 500 times that of cyanide. The molecule was originally synthesized in 1938 by a German company in sight to produce an insecticide, but proved to be more useful in chemical warfare after the German army began testing in 1939.
What makes the use of chemical warfare, subject to such serious allegations? They are weapons of mass destruction that are cheaper, less destructive on the environment, and selective on anyone without a gas mask. So a regime, such as the Syrian army, can use it to eliminate it's citizens, while their army wears protective gas masks. And, as a nuclear blast would demolish a city, a chemical bomb leaves the buildings intact, but with a high death toll. These weapons aren't used to fight a war, but to exterminate an entire population within the desired vicinity. This is why chemical warfare has been banned.
Labels:
chemistry,
current events,
gas,
nerve gas,
news,
phosphorus,
poison,
war,
warfare
Thursday, August 15, 2013
Red Hippopotamus Sweat
Hippos have developed a very interesting form of sweat that possesses additional functionality than cooling down the body temperature. Before studied, the sweat was thought to be blood due to the red colour, but it was found to just be two pigmented molecules which have two functions for the hippo, being a sunscreen, and an antibiotic. It has been found that the main components aren't secreted as most sweat components are, but secreted in subdermal glands, making the classification of the sweat false as sweat is secreted from sweat glands, located in the epidermis.
The two molecules that are secreted are Hipposudoric acid and Norhipposudoric acid (seen below)
These molecules are conjugated systems, meaning in layman's terms that the bonds alternate single to double, but this also means that light is absorbed into this system ranging from the 200nm - 600nm wavelength. This property is what gives the sweat the sunscreen-like properties and the red colour where the Hipposudoric acid is red and the Norhipposudoric acid is orange. As for the antibiotic properties, this is the job of Hipposudoric acid, which inhibits two species of pathogenic bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae.
In finding this, there is an obvious potential for this to be used in consumer sunscreens as there is such a demand for natural sources in cosmetics. Now it's not like there would be a farm somewhere collecting hippo sweat, as there is a method for synthesizing Hipposudoric acid, published in 2006 in Tetrahedron Letters.
Reference
The two molecules that are secreted are Hipposudoric acid and Norhipposudoric acid (seen below)
Hipposudoric acid
Norhipposudoric acid
These molecules are conjugated systems, meaning in layman's terms that the bonds alternate single to double, but this also means that light is absorbed into this system ranging from the 200nm - 600nm wavelength. This property is what gives the sweat the sunscreen-like properties and the red colour where the Hipposudoric acid is red and the Norhipposudoric acid is orange. As for the antibiotic properties, this is the job of Hipposudoric acid, which inhibits two species of pathogenic bacteria Pseudomonas aeruginosa and Klebsiella pneumoniae.
In finding this, there is an obvious potential for this to be used in consumer sunscreens as there is such a demand for natural sources in cosmetics. Now it's not like there would be a farm somewhere collecting hippo sweat, as there is a method for synthesizing Hipposudoric acid, published in 2006 in Tetrahedron Letters.
Reference
Wednesday, August 14, 2013
Minimalist Periodic Table
On my daily roundabouts of the internet I came across this beautifully constructed periodic table of elements created by Alison Haigh. Now this will not be able to get you anywhere as there is no other information on here other than the number of electrons, and you can only obtain that number if you wish to count a bunch of dots. Aside from the practicality point, this is one beautiful work of art that captured my chemist heart. Head to her website to check out the rest of the pictures, or to purchase a print of her work.
Source
Sunday, July 28, 2013
Monocrotophos: A Deadly Insecticide
A recent incident in India has left 23 children dead as a result of cooking oil contaminated with monocrotophos. Being as this was a free meal by government, aimed to keep 120 million children well fed and nourished, this turns some heads.
This molecule has been used as an insecticide, but has since been banned in Canada and the US, along with a number of other countries. They have failed to keep a ban on such pesticides due to the issue of having to feed so many hungry mouths, and needing a high crop output. In the countries where it is still legally used as a pesticide it is required to dilute it to 16% by volume of water. But in this case there was no dilution, indicating that there was direct contact with the poison after the oil processing.
This then becomes a question of who is responsible for insuring this doesn't happen again? One option is to enforce the correct usage and safety for this material, but considering the large illiteracy of the population, and difficulty in setting up an enforcement task, this poses much difficulty.
My opinion in how to prevent something like this from happening again is to enforce limitations on the manufacturer of the insecticide, the ones who enticed the Indian government into lifting the ban with their low price point. To either enforce a ban on synthesizing the compound all together, or to impose a concentration restriction, making the manufacturer dilute before the product is sold
Wednesday, July 17, 2013
The Chemistry of Cement
Cement, the binder used to hold objects together, most commonly used in concrete and mortar. The chemistry of cement can be thought of as a deconstruction of a chemical, only to reconstruct the same chemical, but in a different shape. This molecule that we will be looking at is Calcium Carbonate, a component of seashells, and limestone. To start the process of creating cement, the CaCO3 must be broken down into Calcium oxide through heating it at 825°C, in a process called lime calcination, possessing the equation CaCO3 → CaO + CO2. This Calcium oxide exists in a light powdery form, capable of being transported easily to wherever needs to be cemented. Once the cement is ready to be made, the CaO is mixed with water to produce Calcium Hydroxide, Ca(OH)2, in the equation CaO + H2O → Ca(OH)2. This watery paste is ready to be placed into the mould to set. In the setting process, the Calcium Hydroxide returns to it's original state of Calcium Carbonate, through a slow process of combining with the carbon dioxide within the air in the chemical equation Ca(OH)2 + CO2 → CaCO3 + H2O.
Wednesday, July 10, 2013
Mushroom Coral as a Sunscreen
In an episode of Man vs. Wild that I was watching, a Mushroom Coral's mucus was used as a sunscreen, so me as a chemist interested in cosmetics, I wanted to know what photoprotecting chemicals were in this mucus. The coral Fungia fungites and a couple other species gained this ability to protect themselves because they would grow in shallow water of the Adaman Sea where a lot of UV radiation penetrates through the clear water. First looking at this ability one would expect the coral to synthesize these molecules, but it turns out that the culprits are tiny symbiotic dinoflagellates. In a paper from 1999 by Brown1 studying the makeup of the mucus required for photoprotection, they look at the concentration of xanthophylls in the mucus, the primary photoprotector. Their study looked at how the concentrations of xanthophylls varried throughout the day, and showed that it is best to harvest the mucus at noon when the xanthophylls are at their peak concentration.
So finally to the chemistry of all of this. The molecule shown above is called diatoxanthin, which is a type of xanthophylls, and this is the molecule mostly responsible for filtering out the harmful rays. So how does this happen? Well molecules and atoms absorb light one way or another through absorbing the photons, resulting in electron excitation, and in this case, the molecule absorbs in the UV portion of the electromagnetic spectrum. So what happens after the diatoxanthin molecules become excited? Well biology has found a solution for that, which has been named the Xanthophyll Cycle. It's job is to convert all the used up diatoxanthin back into diatoxanthin.
References:
1. Fitt, W. K., R. P. Dunne, S. W. Gibb, D. G. Cummings, I. Ambarsari, B. E. Brown, and M. E. Warner. "Diurnal Changes in Photochemical Efficiency and Xanthophyll Concentrations in Shallow Water Reef Corals : Evidence for Photoinhibition and Photoprotection." Coral Reefs (1999): 99-105.
Monday, July 8, 2013
4-Methylimidizole, a Pepsi Additive
Recently the news has been discussing Pepsi's additive 4-methylimidizole in the caramel colouring of their popular drinks, with the fact that the Centre for Environmental Health has deemed it unsafe. Now should you go into your fridge right now and toss all of your Pepsi into the garbage? No. Although a study that was performed in 2007 gave results showing that 4-methylimidizole has carcinogenic properties in mice and rats, keep in mind that this is at an extremely high dose (115mg/kg of body weight) and the dosage that a normal consumer will receive showed no effects.
So why is this molecule in Pepsi in the first place? It comes with the additive caramel colour, which is produced by a separate manufacturer and is listed as Caramel Colour on the ingredients list. This molecule is made as a product of the Maillard Reaction, the browning of food, and in this case being the reaction to produce the caramel colour. But the problem with their method in producing the caramel colour is that they use a method with a higher concentration of ammonia, which incidentally ends up producing more 4-methylimidizole than other methods. Since this has come out, the US caramel colour manufacturers have changed their recipe to adhere to the people's reaction, lowering the overall concentration of 4-methylimidizole. This has yet to change the formulation in other parts of the world though.
Labels:
carcinogen,
chemistry,
current events,
drink,
food,
food chemistry,
maillard,
news,
organic,
pepsi
Thursday, June 27, 2013
How the Internet is Changing Your Brain
I was introduced to this video on Academic Earth in relation to my post about where science education should go, and it goes without saying that they harmonize each other very well. Please watch this 2 minute video, and it will open your eyes to what our technology is doing to our brains.
Created by AcademicEarth.org
Created by AcademicEarth.org
Tuesday, June 11, 2013
Why do Onions Make You Cry?
Cutting onions is arguably the worst part of cooking, the burning irritation you get in your eye becomes unbearable so much as to take a break mid way through chopping. So why is this? This is all to do with mixing molecules in the onion cells that aren't supposed to be together, an enzyme named Allilinase and a molecule called Allilin. Think of this like the type of adhesive that has two parts that you need combine together to make the glue active. When we cut onion we start the chain of reactions allowing the Allilin to come in contact with Allilinase to turn the Allilin into Sulfenic Acid and this Sulfenic Acid turns into syn-Propanethial-S-oxide though the enzyme Lachrymatory Factor Synthase.
This syn-Propanethial-S-oxide is what everyone hates because this is the irritant that when combined with the moisture in your eyes gives you that pain and your body tells your tear ducts to try and wash it out.
References:
Something about Science
Why Does Garlic Sometimes Turn Blue?
I've noticed sometimes when I cook garlic in butter that the garlic will turn blue after a while, and I had to ask myself why this happens. After finding out that it is safe and poses no loss of taste, I sighed and continued reading. It seems to be due to the presence of sulfates within the garlic, a component of what gives it such a pungent smell. What happens is that the sulfates combine with the copper ions present in the butter, water, or cooking equipment to create something known as Copper (II) sulfate, or CuSO4. This new compound is what gives off that bright blue colour.
So you may ask now if there are any precautions to be taken to avoid receiving this colour. Luckily science has gained us much information about this process. The main component of this phenomena is an enzyme present within the garlic that catalyzes this reaction, and can be deactivated simply by heating at high temperatures, or by letting the garlic age.
Monday, June 3, 2013
Hydrangeas and their Variety of Colour
Hydrangeas (Hydrangea macrophylla) have been known to form in a range of colours, from red, to purple, to blue, with having nothing to do with the genetics of the plant. So, if this colour difference isn't due to the genes, it must be due to certain environmental factors, and as it turns out, it is all to do with the pH and other properties of the soil. The more acidic the soil, and the greater [Al3+] in the soil, the more blue the sepals of the hydrangea will become. So why is this? Let's start with looking at the pigments which govern the colour of the sepals. The major pigment found in Hydrangeas (along with a number of other plants) is called Myrtillin, also known as delphinidin 3-glucoside, which is part of a group of molecules called anthocyanin.
But odly, this molecule assumes a red colour in acidic conditions and blue in basic conditions1 (opposite to that of the soil conditions) and the pH within the sepals is usually slightly acidic, with little variation, so the blue colouring must have something to do with the Al3+. Through much research, Kondo et al. 2, came up with a model proposing that the aluminum acts as a bridge to coordinate the Myrtillin to other copigments pigments known as acylquinic acids, which produces this blue colour.
While this is how the blue colour is formed, it can be safely assumed that the red colour is merely caused by Myrtillin by itself without any co-pigments or metal chelation. A question which hasn't been answered yet is what the pH of the soil has to do with all of this. The low pH allows there to be more free aluminum ions within the soil, to allow there to be more [Al3+] taken up into the plant.
References:
Second figure from 1.
1. K. Yoshida, M. Mori and T. Kondo, Nat. Prod. Rep., 2009, 26, 884–915.
2. T. Kondo, Y. Toyama-Kato and K. Yoshida, Tetrahedron Lett., 2005, 46, 6645–6649.
But odly, this molecule assumes a red colour in acidic conditions and blue in basic conditions1 (opposite to that of the soil conditions) and the pH within the sepals is usually slightly acidic, with little variation, so the blue colouring must have something to do with the Al3+. Through much research, Kondo et al. 2, came up with a model proposing that the aluminum acts as a bridge to coordinate the Myrtillin to other copigments pigments known as acylquinic acids, which produces this blue colour.
While this is how the blue colour is formed, it can be safely assumed that the red colour is merely caused by Myrtillin by itself without any co-pigments or metal chelation. A question which hasn't been answered yet is what the pH of the soil has to do with all of this. The low pH allows there to be more free aluminum ions within the soil, to allow there to be more [Al3+] taken up into the plant.
References:
Second figure from 1.
1. K. Yoshida, M. Mori and T. Kondo, Nat. Prod. Rep., 2009, 26, 884–915.
2. T. Kondo, Y. Toyama-Kato and K. Yoshida, Tetrahedron Lett., 2005, 46, 6645–6649.
Wednesday, May 22, 2013
How Bioluminescence Works
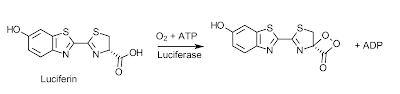
Bioluminescence is the ability of a living organism to emit light; a phenomenon which has intrigued humans for thousands of years and allowed them to invent mystical and magical stories behind these beacons of light. We might have decided that these lights serve a magical purpose, but evolution has found many uses for this process from protection, all the way to communication. The chemical mechanism behind these bioluminesent photons is a very simple one, though not the same in all organisms, they share some of the same basic principles. Taking the firefly as an example, the compound which acts as it's light bulb is called luciferin, and the reaction begins with molecular oxygen and ATP, with the help of the enzyme luciferase, turning luciferin into a dioxetane derivative (mechanism of this can be seen here)1.
Once this dioxetane derivative is formed the emission of light reaction is ready to occur spontaneously. This happens in a concerted reaction releasing CO2 and producing an excited ketone. The electron of the excited ketone then falls in energy to create a photon and end with the final product of oxyluciferin.
The newly created oxyluciferin is then enzymatically recycled into luficerin by this mechanism here2.
References:
1. Aldo Roda Chemiluminescence and Bioluminescence: Past, Present and Future, p. 57, Royal Society of Chemistry, 2010, ISBN 1-84755-812-7
2. Keiko Gomi and Naoki Kajiyama. Oxyluciferin, a Luminescence Product of Firefly Luciferase, Is Enzymatically Regenerated into Luciferin. J. Biol. Chem. 2001 276: 36508-36513. July 16, 2001, doi:10.1074/jbc.M105528200
The newly created oxyluciferin is then enzymatically recycled into luficerin by this mechanism here2.
References:
1. Aldo Roda Chemiluminescence and Bioluminescence: Past, Present and Future, p. 57, Royal Society of Chemistry, 2010, ISBN 1-84755-812-7
2. Keiko Gomi and Naoki Kajiyama. Oxyluciferin, a Luminescence Product of Firefly Luciferase, Is Enzymatically Regenerated into Luciferin. J. Biol. Chem. 2001 276: 36508-36513. July 16, 2001, doi:10.1074/jbc.M105528200
Labels:
atp,
biology,
bioluminescence,
enzyme,
excited ketone,
firefly,
hv,
ketone,
light,
organism,
peroxide
Sunday, May 19, 2013
Why are Flamingos Pink?
This is a question that many children will ask their parents at some point, and if the child is chemically literate this is what the parents could tell them. Flamingos primarily eat crustaceans and algae from their aquatic environment, which we usually see them standing in, and in their food, there is what is known as carotenoid pigments. Carotenoids are made from fats, and the incorporated into the chloroplasts of algae to serve as light absorbing pigment. Then the algae get eaten by the crustaceans or flamingos, the carotenoids get passed on into these animals. Usually when a molecule is eaten, it breaks down, but something such as a pigment will absorb into your bloodstream and not break down, so when a flamingo eats these, the pigments enter the blood stream and then eventually come out in their feathers. Now for flamingos, the primary pigments which get absorbed and end up in their feathers, are Canthaxanthin (the molecule below), astaxanthin and phoenicoxanthin 1. Canthaxanthin is a molecule which gives of a bright violet colour when it is crystallized but when it is incorporated into flamingo feathers, it is diluted to give a vibrant pink.
References:
1. Fox, D., and T. Hopkins. "Comparative Metabolic Fractionation of Carotenoids in Three Flamingo Species." Comparative Biochemistry and Physiology 17.3 (1966): 841-56.
Tuesday, April 23, 2013
Ricin: A Potent Poison
Now I don't really talk about anything on this site that deals with anything biochemistry related, but I will make this an exception due to the state of current events. Ricin might be a word that you have heard recently in the new, about the poising attempt on President Obama by an Elvis impersonator. For those who don't know what Ricin is, it is a protein originating from the same plant that gives you castor oil. It's job in the body is a nasty one, as it inhibits protein synthesis (which is pretty much everything important in a cell), more specifically it acts upon the ribosome (a small organelle which synthesizes proteins). It's categorization of inhibitor is a Ribosome Inactivating Protein, which cynically has the acronym RIP. Although this is a very deadly poison (a gram worth to kill), there does exist an antidote, but the survivors will have major organ damage and will have a shortened life expectancy.
Source:
Wikipedia
Tuesday, April 9, 2013
Biology 12 BC Study Guide
I just realized that I still have my ginormous study guide that I made back in high school when I was determined to get 100% in biology (of which I didn't achieve unfortunately) because I was certain that I wanted to go into medicine. I'm not joking when I say this thing is large, it is 31 pages long, and now you must think "Wow, this guy was really cool in high school, spending his friday nights making study guides". It is true that I would compulsively make elaborate study guides, mainly for the sake of keeping all of my information organized. Enough of the bantering of my crazy educational antics, here is the study guide:
BC Biology 12 Study Guide
I wish luck to all of you high schoolers who download this!
BC Biology 12 Study Guide
I wish luck to all of you high schoolers who download this!
Thursday, April 4, 2013
Should Search Engines Block Queries to The Anarchist's Cookbook?
This thought came to me when I decided
to search for The Anarchist's Cookbook to
calm my curiosity of how easy it is for anyone to get a hold of it.
When I searched Google for it, the first page presented me a number
of places where I could purchase the book, along with a full pdf file
of the book. I was quite surprised when I found it
so quickly, and quite
disturbed at that as well.
I am fully against internet censoring to the extent of websites
being shut down (other than that of illegality issues), but I believe
that search engines such as Google, Bing, and Yahoo should take a
step towards assuring that the average internet user cannot obtain
information
like this.
The
problem that I have with The Anarchist's Cookbook
is not the fact that it explains how to make explosives, I think it's
good information for people who know what they are doing, such as
chemists, but I have a problem with the fact that it is written for
the average person, who has had no safety training in handling
chemicals. It explains how to get certain chemicals from off the
shelf products in order to construct bombs, and other such dangerous
weapons, rather than having
to get chemicals from a supplier such as Sigma-Aldrich, who makes it
impossible for the average person to obtain.
If someone really wanted to
hurt someone, they should have to gain the knowledge first before
jumping right to making the explosive from a set out procedure. In
that time planning it out, they could very well change their mind.
An example of this is the Drano Bomb, where kids can make a bomb out
of common household items, which can cause serious damage, as
reported here.
Reports have been made of people loosing fingers while holding the
water bottle, which is an unexpecting object to be a bomb since we see bottles littered all the time.
In the
end, we have to ask ourselves if information like this should be free
to the public, through being written in layman’s terms, rather than
in academic terminology that could only be understood with four years
of post secondary education, along with the understanding and
appreciation of what we are dealing with.
Labels:
bombs,
censor,
chemistry,
drano,
drano bomb,
explosives,
illegal,
morals,
opinion,
search
Wednesday, April 3, 2013
Ponderings Of Where Science Education Should Go
Just
as a forefront, I don't want to make myself out to be an expert in
the educational studies; these are merely my observations and
thoughts of where I believe we should be heading with our public
elementary and high schools.
A
step back from memorization
A
key part in education right now is the preparation for standardized
tests, to reshape a student to be able to answer predictive
questions, selecting students who aren't well rounded, but excel
solely in this category, while leaving their creativity behind. This
method doesn't teach children to ask questions because they soon
realize that there is only one right answer, where in the real world
a person needs to ask themselves questions in order to problem solve,
or to see a problem all together. I can't tell you the number of
times I have heard an arts student say that they didn't like science
because there was only one right answer, and that they preferred to
have an objective look on their topics. But this here, is the beauty
of science; anyone can disprove anything that is considered “true”
with sufficient evidence, creating a paradox in how we test the
subject. If education can step back from the emphasized learning of
knowledge and spend more time exploring the beauty of the scientific
method, people won't be as afraid of science. I'm not necessarily
saying that I think everyone should go into the sciences because of
this, but people who are in other fields shouldn't have a fear of it.
In
line with this, there may also be a problem with this method for the
upcoming generation who are surrounded with more and more electronic
gadgets connected to all human information. With an increasing
dependence on technology, the need of memorizing small details
diminishes proportional to the easiness of accessing the information
[1]. In fact, it has been proven that if the information is easily
accessed, our mind will refrain from storing it in our long term
memory. From this, we may see a change with our next generation in
their ability to hold long term memories of small facts. Because of
this, we need to push for a stronger investment in the creativity and
imagination of students. It was Einstein who stated that "Imagination
is more important than knowledge. Knowledge is limited. Imagination
encircles the world”.
Consequences
of the current education system
One
key result of this is the fact that 3rd
year
genetics is considered to be one of the toughest courses for biology
undergraduates, but
for other majors such as mathematics, physics, and chemistry it seems
easier because of their problem solving experience.
Up
to that point in university biology students are
made to only know memorization as the medium of education. They
aren't made to problem solve, create, or learn the scientific method
until later in their degree; all they are doing is building up their
knowledge to be able to problem solve later. As much as this method
has worked for years and years, I believe
that we can do better in educating students so that when they do get
to their first genetics class, they have the problem solving skills
to be able to excel in the course.
As
have just finished my undergrad, i have witnessed a slew of grad
students who eagerly boast in their academic excellence, while in
their T.A. duties saying things such as "Wow, you don't know
that", and refuse to teach an elemental concept. This power trip
blocks the process of education; being in an employee of an academic
institute, they are there to help educate and build the body of
science, but instead think selfishly in looking at their academic
achievements. They are the kind of people who were bred out of this
academic competition that exists in a capitalistic society, a concept
of every man/woman for themselves, only yielding the best of the
best. But these people fail to see what science really is. They fail
to see that it is a body of knowledge that is dependent of everyone
working as a whole.
Ending
Comments
It
will be tough to be subjective through a land of no standardized
tests, creating a lot of issues in how to test the mass number of
students without multiple choice. The recourses to mark thousands of
objective tests at a time are just not here unless we can find a
testing method in the future that both appeases this method along
with the practicality of marking.
I'm
not necessarily saying that we should put all of our teaching into
the creativity process because we will still need to have memorized a
great deal to get anywhere in the scientific world. Our brains rely
on connections to get anywhere, which requires us to have this
knowledge in our memory. We need to see the comparisons that the
computers are unable to see, this also being known as human
intuition.
References
[1]Sparrow, Betsy, Jenny Liu, and Daniel Wegner. "Google Effects on Memory: Cognitive Consequences of Having Information at Our Fingertips." Science333.6043 (2011): 776-778.
Thursday, March 21, 2013
Internet Detox
I have decided to take a break from the internet for a week. This is because I have made a really deep addiction to constant flowing information and I need to disconnect myself from this addiction. It all stems from the last year, being stuck in bed with only one outlet to the outside world.
Any post that I make in the next week will be on my phone, as all I will limit myself to is email, and this blogger app.
Wish me luck
Any post that I make in the next week will be on my phone, as all I will limit myself to is email, and this blogger app.
Wish me luck
Friday, March 15, 2013
Antoine Lavoisier's Understanding of Caloric and the Properties of Water
The process of science is a wonderful thing, which is what drives me into trying to understand the history of science. My recent dive into this is reading Antoine Lavoisier's Elements of Chemistry, as it is one of modern chemistry's first textbook written by one of it's fathers. Now this isn't the easiest piece of literature to read, not because of the language, it excels in that category, but the font chosen makes the s's appear as f's. Difficulties aside, here is a quote I fell in love with, showing his understanding of the cohesion of water molecules before there was an understanding of hydrogen bonds, and heat before there was an understanding of molecular kinetic energy.
Just as a background fact, Caloric was Lavoisier's term for kinetic energy, and at that time was considered to be a fluid or gas that would flow through matter, making it either hot or cold. Just as heat flows from hot to cold, Caloric would flow from a hotter material to a cooler one until it reaches an equilibrium. All of these observations, now obvious to us, were new and exciting for the scientists at the time, and to think there was a fluid that flows dictating temperature wasn't far off from the truth considering that matter with more kinetic energy expands according to PV = nRT.
It is, perhaps, more natural to suppose, that the particles of caloric have a stronger mutual attraction than those of any other substance and that these latter particles are forced asunder in consequence of this superior attraction between the particles of the caloric, which forces them between the particles of other bodies, that they may be able to reunite with each other. We have somewhat analogous to this idea in the phenomena which occur when a dry sponge is dipt into water: The sponge swells; its particles separate from each other; and all its intervals are filled up by the water. It is evident, that the sponge, in the act of swelling, has acquired a greater capacity for containing water than it had when dry. But we cannot certainly maintain, that the introduction of water between the particles of the sponge has endowed them with a repulsive power, which tends to separate them from each other; on the contrary, the whole phenomena are produced by means of attractive powers; and there are, first, The gravity of the water, and the power which it exerts on every side, in common with all other fluids; 2dly, The force of attraction which takes place between the particles of the water, causing them to unite together; 3dly, The mutual attractions of the particles of the sponge with each other; and, lastly, The reciprocal attraction which exists between the particles of the sponge and those of the water. It is easy to understand, that the explanation of this fact depends on properly appreciating the intensity of, and connection between, these several powers. It is probable, that the separation of the particles of bodies, occasioned by caloric, depends in a similar manner upon a certain combination of different attractive powers, which, in conformity with the attractive powers, which, in conformity with the imperfection of our knowledge, we endeavour to express by saying, that caloric communicates a power of repulsion to the particles of bodies.
I recommend this read to anyone interested in the history of our chemical body of knowledge, or to show respect to the people who worked undyingly hard to make these pivotal discoveries.
References:
Lavoisier, Antoine Laurent. Elements of Chemistry, in a New Systematic Order, Containing All the Modern Discoveries. New York: Dover Publications, 1965. Print.
Wednesday, March 13, 2013
For Those Students Who Have Trouble in Drawing Tetrahedrals
Just a relay of a blog post I found on Master Organic Chemistry explaining the difficulties in viewing 2D representations of the Tetrahedral.
Find it Here.
Find it Here.
When the Catholic Church and Science Get Along
I asked myself yesterday, while watching TV, what is used to make the papal smoke turn either white or black, and how do they make sure there is no false alarm. In my research I found that they previously used wet straw to make the black smoke and nothing added to make the white smoke. But in an article by The Guardian, I found that they now use an electronic smoke machine, starting in 2005, where potassium perchlorate (KClO4), anthracene (3 fused benzene rings), and sulphur is used to make the black smoke, while potassium chlorate (KClO3), lactose and a pine resin is used to make the white smoke.
So how does this work?
Black
The anthracene (from tar) helps the burnt carbon from the paper flow upwards in the smoke instead of remaining in the ash. The KClO4 is used to aide the combustion of the paper to create the chunks of carbon. What carries all of this upward is the water that is generated in the chemical reaction of combustion. Just think of it as the burnt carbon as being graphite, which produces a back colour.
White
What creates the white smoke is the existence of unburnt paper, or other unburnt fuel, rising up with the heat. The paper is thermally broken apart in a process called pyrolisis, leaving small white particles of unburnt cellulose, or from the added resin, rising up as the combustion product of H2O rises out of the chimney. Just think of the particles of unburnt fuel being like table sugar, which gives off a white colour.
References:
Via: The Guardian
Labels:
application,
catholic church,
chemistry,
colour,
fact,
history,
pope,
smoke
Tuesday, March 12, 2013
Egyptian Blue: Calcium Copper Silicate
In a recent article by Scientific American, a story is told of Calcium Copper Silicate (Egyptian Blue) being the first artificial pigment made by humans, and soon to be utilized, once again, as a nano ink for biomedical imaging. I'm not going to go over the details of the article, but I will present some background information of this soft blue colour.
Being the first recorded synthetic pigment, you may ask how it was originally synthesized. The first procedures, in 3000 B.C., involves the heating of sand, calcium carbonate, copper, and an alkali substance to obtain this blue pigment having a composition of CaCuSi4O10. The reaction below is a general understanding of the reaction, where the molecules can be variable in a number of different ways.
References:
Via: Scientific American
Wikipedia
Being the first recorded synthetic pigment, you may ask how it was originally synthesized. The first procedures, in 3000 B.C., involves the heating of sand, calcium carbonate, copper, and an alkali substance to obtain this blue pigment having a composition of CaCuSi4O10. The reaction below is a general understanding of the reaction, where the molecules can be variable in a number of different ways.
Cu2CO3(OH)2 + 8SiO2 + 2CaCO3 → 2CaCuSi4O10 + 3CO2 + H2O
The blue colour comes from the absorption of the copper, which can range from the blue, seen above, to a much darker blue, depending on how pure the mixture is. But what wasn't known at that time, was that when irradiated with visible light, the compound emits IR photons, which is the property that will conduct the imaging technologies.
References:
Via: Scientific American
Wikipedia
Monday, March 11, 2013
What is Triclosan and Why is it Being Banned?
Triclosan has been in the media recently, in it's battle with regulation in its existence in many cleansing products. In a recent article by the C&EN News they reported that "Minnesota state agencies will be able to purchase only soaps and detergents that are free of the antibacterial ingredient triclosan. State officials say they are concerned that the chemical has been linked to endocrine disruption and the growing threat of antibiotic resistance."
What is Triclosan, and how long have we been in contact with this reportedly "harmful" chemical?
It has been used commonly in many different products since 1972 when it first became apparent that it reduced bacterial activity. Such products as toothpaste, mouthwash, deodorant, trash bags, kitchen tools, and many other areas where antibacterial products seemed appropriate for manufacturers to use for preserving their products, or making their cleaning products more affective.
Why is it being banned?
It is being banned for a number of reasons. First of all, as mentioned by C&EN, the risk of upsetting endocrine function. This hasn't necessarily been proven in human subjects (other than slightly affecting the immune system, which may be due to the vast number of bacteria in, and around the body 1), but it has been reported that the North American Bullfrog's endocrine system becomes compromised under low doses of Triclosan 2. Another reason is that it has been proven that it decreases bacterial species diversity, in other words, selects for antibiotic resistance. The third reason is that it can be harmful to the environment for the reasons stated above (i.e. disrupting aquatic bacteria), along with Triclosan's byproducts such as dioxins being harmful.
How to avoid using products with Triclosan?
If you don't wish stay free of this product, read labels, and educate yourself of what is in the products you are using. It may also be listed under trade names of UltraFresh, Amicor, or BioFresh.
References:
Via: C&EN
1. Erin M. Rees Clayton, Megan Todd, Jennifer Beam Dowd, Allison E. Aiello. "The Impact of Bisphenol A and Triclosan on Immune Parameters in the U.S. Population, NHANES 2003–2006". Environ Health Perspect 119 (3): 390–396. doi:10.1289/ehp.1002883
2. Nik Veldhoen, Rachel C. Skirrow, Heather Osachoff, Heidi Wigmore, David J. Clapson, Mark P. Gunderson, Graham Van Aggelen and Caren C. Helbing. "The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development". Aquatic Toxicology 80 (3): 217–227. doi:10.1016/j.aquatox.2006.08.010
Friedel-Crafts Alkylation
The Friedel-Crafts Alkylation reaction is used to add an alkyl group to an aromatic ring in the presence of a strong lewis acid. This reaction was developed by Charles Fiedel (France) and James Crafts (USA) in 1876, in studying reactions involving the creation of carbon-carbon bonds, some of the most valuable technology in organic chemistry.
This reaction involves taking an aromatic ring and adding an alkyl halide (Cl-R1) with a strong lewis acid (AlCl3), where the alkyl halide can exist as a tri-substituted, all the way to a methyl. The chemistry of this reaction involves the AlCl3 to strip the Cl from the alkyl halide, forming a carbocation (R+), followed with the π electrons of the aromatic ring to grab the carbocation. The AlCl4- can now strip the H away from the position where the R added on the aromatic ring, reverting the catalyst back to it's original form, and the final product to be formed.
Trial of MarvinSketch, a Free ChemDraw
I've been hidden away from the world the past couple of weeks as I've been having to take care of my grandmother, and now I have found some free time so I thought I would download MarvinSketch to try it out. I have been meaning to try it for quite sometime, as I've been interested in all the chemical drawing programs out there. Currently, I have been using ChemSketch by ACD Labs, and has shown it's exceptional quality over the past year, and before that I had been using ChemDoodle, which has been the best program I have tried so far, but unfortunately I was forced into changing ecosystems from mac into windows, leaving it unused. I recommend trying ChemDoodle as a cheap alternative to ChemDraw, and plus, they are creating some neat HTML5 powered technology, which I use when I need to make a quick sketch.
But I will be downloading this Java based sketcher, which does look somewhat complicated, but I will do a review post once it is up and running.
Update: I was just informed that I can receive another activation code for ChemDoodle to add to my second computer. I recommend this product to anyone looking for a ChemDraw alternative, as it contains all of the current capabilities at a small fraction of the price. And as seen here, they have unmatched support for their products. Sorry for it sounding as though I am advertising, but I am truly amazed with their services.
But I will be downloading this Java based sketcher, which does look somewhat complicated, but I will do a review post once it is up and running.
Update: I was just informed that I can receive another activation code for ChemDoodle to add to my second computer. I recommend this product to anyone looking for a ChemDraw alternative, as it contains all of the current capabilities at a small fraction of the price. And as seen here, they have unmatched support for their products. Sorry for it sounding as though I am advertising, but I am truly amazed with their services.
Labels:
Chemdoodle,
Chemdraw,
chemistry,
doodle,
draw,
drawing,
internet,
marvinsketch,
programs,
sketch
Saturday, February 23, 2013
2/23/2013 - Soap
To start off, the formal definition of a soap is a water-insoluble fatty acid mixed with an organic base or an alkali metal, which produces a carboxylic acid salt, and in-turn increasing the solubility in water. Although they technically are chemically modified, they are usually not considered synthetic. Soaps have been dated back to at least 2300 years, possibly originating from the Celts, and their original procedure for creating these soaps was to boil animal fat in water with some form of ash, usually from wood. This saponifies the fat to the free fatty acids. The chemical in the ash that is actively turning this fat into soap is potassium carbonate (K2CO3), which first deprotonates the fatty acid producing a bi-product of HCO3, while the potassium acts as a counter ion, producing the salt.
This carboxylic acid salt can interact with water now since it's head is more charged, rather than an uncharged fatty acid. The way this soap now works is that the long carbon chain interacts with oil (from skin, hair or any other source on your body), while the charged end interacts with water allowing the oils to be washed away with an excess of water.
References:
Myers, D. Surfactant Science and Technology, 3rd ed. Wiley-Interscience: New Jersey, 2006.
This carboxylic acid salt can interact with water now since it's head is more charged, rather than an uncharged fatty acid. The way this soap now works is that the long carbon chain interacts with oil (from skin, hair or any other source on your body), while the charged end interacts with water allowing the oils to be washed away with an excess of water.
References:
Myers, D. Surfactant Science and Technology, 3rd ed. Wiley-Interscience: New Jersey, 2006.
Labels:
alkali metal,
chemistry,
cleaning,
fact,
fat,
fatty acid,
history,
organic,
organic chemistry,
potassium,
soap
Monday, February 11, 2013
2/11/2013 - Magnesium Oxide
This post isn't so much a reaction, but a little informational blurb on magnesium oxide, something that is involved in our everyday life that we don't know about. One of it's major jobs on our planet is as a refractory material, or known to the non-engineering world, as something that can hold something else that is extremely hot! It is used as a refractory material because of it's unusual chemical and thermal stability (i.e. it won't react or melt under high temperatures), because it's melting point is 2852°C, which is about half as hot as the earth's core. In my undergrad thesis I had the pleasure of working with this substance in the crucibles for TGA (Thermogravimetric Analysis) experiments, because the crucible needed to handle hot temperatures. Now industrial heat isn't all MgO can handle, it is also known to medically relieve Heartburn, along with sore stomach and diarrhea, as acting as an antacid. As for the chemistry of this substance, it is difficult for it to react with anything, but to make it it involves the calcination (heat under oxygen) of Magnesium hydroxide or Magnesium carbonate. Overall, this is a substance that would be hard to live without in today's world and has already gained my respect!
Image source
Info Source
Image source
Info Source
Labels:
engineering,
magnesium,
magnesium oxide,
medical,
Mgo,
refractory,
tga
Monday, February 4, 2013
2/4/2013 - Prasad Reduction
This was a reaction that I had learnt in my third year organic chemistry course and has been emphasized quite a bit as a very powerful reaction to control stereoselectivity in reductions. The Prasad Reduction, first developed in 1984, uses a boron chelating agent to tether the ketone and alcohol together, creating a favored boat transition shape, which is what drives the selection of reduction as seen below. The reducing agent in this case is sodium borohydride, which attacks the most favorable site of reduction, which can be determined through a simple model of the boat transition state.
Image: Wikipedia
Friday, February 1, 2013
Organic Chemistry Cook Book
Last year I had decided to start typing up my old second year organic chemistry cook book so that I would have easy access to it whenever I needed to reference a basic equation. Since I no longer need this I have decided to make revisions and make it available to anyone online to help them with their second year organic chemistry courses. My professor back in second year highly recommend that we make this "CookBook" as we go a long and learn each reaction, and ever since that course I have referred to it for any reaction I had forgotten or just needed some hints on how to go through a synthesis problem.
I'm going through it pretty slowly as I don't have that much time to work on it, but here is what I have so far.
ORGANIC CHEMISTRY COOKBOOK
And please if you want to add any obscure reactions to it, please comment below to let me know.
I'm going through it pretty slowly as I don't have that much time to work on it, but here is what I have so far.
ORGANIC CHEMISTRY COOKBOOK
And please if you want to add any obscure reactions to it, please comment below to let me know.
Thursday, January 17, 2013
1/17/2013 - McMurry Coupling
Hello Chemists, I'm sorry that I have been absent for so long on this blog. This last term posed more difficult than initially anticipated since I also had my recovery to worry about. But now that I have finished with school I can solely focus on my very frustrating recovery.
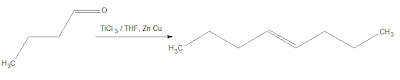
For my first reaction of the new year I have decided to look at a coupling reaction that I worked with in my previous semester, McMurry coupling. This reaction is named after John McMurry, the man who wrote my second year organic chemistry textbook, of which I always reference in those times of a brain blank. This reaction is a technique used to couple two ketone functional groups together to form a bridged olefin across those two molecules. This is done through the addition of TiCl3 in THF with Zinc and Copper.
Subscribe to:
Comments (Atom)