I thought I would have had time to post some reactions but being back at school, combined with my handicap only leaves me exhausted, tired, and sore once I get home. But here I present the molecule Amygdalin. I am currently reading this spectacular book Guns, Germs, and Steel (I definitely recommend it), which is about the evolution of civilization and why different civilizations grew to dominate over others. The author Jared Diamond talks about how crops were domesticated and noted that wild almonds are toxic, so I decided to investigate what the toxic component was. And that brought me to this molecule. The metabolism of this molecule releases that Cyanide group, creating HCN, making it very toxic to humans. This has been used in a number of cancer treatments, and subsequently derived into other molecules to enhance the effect.
Monday, September 24, 2012
Tuesday, September 4, 2012
Starting a New Semester
I'm starting my last semester (the semester that should have been last year but unfortunately some idiot drove his mini van into me) tomorrow. I'm very excited to finally finish this very prolonged degree, but it is also stressful in a way because I really can't choose between going to grad school or going into industry straight off (where before it was grad school, grad school, grad school), either way I am aiming on going into the Cosmetics industry. This website here has really inspired me into learning more and more about cosmetics and the chemistry behind it. For my last semester I am taking Natural Synthesis (chemistry of natural chemical reactions), Bio-inorganic chemistry, and Food Chemistry. So expect many future posts to be related to these topics.
Sunday, September 2, 2012
9/2/2012 - Cleaning Glue Goop
Recently I decided to take off a sticker that I applied to my laptop a while ago, but unfortunately it left a pretty icky residue that I couldn't seem to remove. So my girlfriend suggested that I buy Glue Gone, but me being me I decided it would be a good idea to investigate what exactly Glue Gone is. It came apparent that it was made of various natural oils (particularly citrus oils) and works by the means of dissolving the bonds that the adhesive has made to the surface. This leaves the goop floating on a thin layer of oil and now easy to remove. So in my ecstatic excitement of learning something new I decided to try and remove the adhesive with olive oil with a tiny drop of lemon juice. This method worked wonders in it's efficiency as I was finished cleaning in under a minute. So for all of those DIY cleaning products people out there, here is your technique for cleaning residual adhesive.
Monday, August 27, 2012
8/27/2012 - Chocolate's Caffiene
The cacao plant has many alkaloids within it's beans, but the most prominent regarding their affects on human physiology is the molecule Theobromine. Cacao does contain a small amount of caffeine, but Theobromine tends to be the prominent stimulant. Theobromine and caffeine are part of a class of alkaloids called methylxanthines, and the liver's metabolism of caffeine actually involves the breakdown of caffeine into Theobromine, and then subsequently into various other molecules. Theobromine is used medically in asthmatic cases due to it's ability to loosen the muscles in the bronchus, but too much of this molecule is poisonous due to this affect. This ability also makes it quite poisonous to animals such as dogs (due to the slower metabolism of the molecule), making the Christmas season dangerous for when your dog decides to eat all your chocolate.
 |
| Theobromine |
 |
| Caffeine |
Sunday, August 5, 2012
Congratulations NASA
You continue to make great steps in the name of science. Here is the first image broadcasted by Curiosity:
Monday, July 23, 2012
7/22/2012: Bleach
Now everyone is familiar or should be familiar (for those who don't clean) with bleach. Bleach is a chemical known for its cleaning capabilities and its ability to remove pigments or colour from objects, and its disinfectant ability. Bleaching is a general form and consists of a couple different methods, but in particular I will be talking about the use of Sodium hypochlorite (NaOCl) being that it is the most popular. The power of this chemical comes from it's oxidizing ability when it combines with water to make HClO, since it is a molecule of high electronegativity. The oxidation process goes as follows 2HClO(aq) + 2H+ + 2e- → Cl2(g) + 2H2O . There has also been very extensive research in it's disinfectant process. The first synthesis performed by Claude Louis Berthollet in 1789 is very simple where it consists of passing chlorine gas through sodium hydroxide:
Cl2(g) + 2NaOH (aq) → NaCl (aq) + H2O (l)
Thursday, July 12, 2012
07/12/2012 - Organolithium Ether Cleavage
There comes a point in a compound's life when there is a question of whether there is an easier way to make it, and in 1930 Karl Ziegler questioned this with organolithium compounds. Not only did he find more simpler ways of preparing such organolithium compounds but he developed many applications for the use of the light metal. The largest application of these compounds is in organic chemistry as organolithium reagents, used in creating those precious carbon-carbon bonds. But one of the first applications that Ziegler developed was the ability of Lithium to cleave an ether bond, demonstrated in the following reaction:
PhCH2OMe + 2Li → PhCH2Li + MeOLi
Wednesday, July 11, 2012
7/11/2012 - Generation of Alkyl Radicals
The next year in 1929 one of the pioneers of radio chemistry, Friedrich Adolf Paneth, an Austrian scientist who did the majority of his early work at Königsberg University, completed a journey set out by Sir Edward Frankland. This discovery gained humanity the ability to create alkyl radicals, and the identification of such radicals through their ability to displace a metallic mirror (silver mirror etc.). This was performed through the pyrolysis (thermal decomposition) of a lead organometallic with the general formula of PbR4.
Tuesday, July 10, 2012
7/10/2012 - Beginnings of Carbonyl Chemistry
In 1928 a chemist by the name of Walter Heiber, also known as the father of carbonyl chemistry, started a systematic study of many metal carbonyls. This allowed him to discover many of the hidden catalytic properties of these utilizable compounds. Two of his most famous reactions were in the realm of Iron chemistry with the two following reactions:
Fe(CO)5 + H2NCH2CH2NH2 → (H2NCH2CH2NH2)Fe(CO)3 + 2CO
Fe(CO)5 + X2 → Fe(CO)4X2 + CO
Fe(CO)5 + X2 → Fe(CO)4X2 + CO
Monday, July 9, 2012
7/9/2012 - Synthesis of Tetraethyllead Pt. II
In the year of 1922 two chemists working with General Motors Corportation Thomas Midgley and T.A. Boyd discovered an additive to gasoline that would prevent engine knocking, meaning uncontrollable combustion. This additive was Tetraethyllead, an organometallic discovered back in Germany in 1854 but not utilized until this point. This great discovery 'lead' to the creation of new gasoline solutions which have since phased out due to the high toxicity of lead in the exhaust fumes. The synthesis of Pb(C2H5)4 is performed through the following reaction of a sodium lead alloy with chloroethane:
4NaPb + 4CH3CH2Cl → (CH3CH2)4Pb + 4NaCl + 3Pb
Sunday, July 8, 2012
7/8/2012 - Synthesis of Polyphenylchromium Compounds
The past couple of days I have been recovering from my dentist reaching into my mouth and stealing my precious wisdom teeth, and dealing with my new found tolerance to most pain killers making it much more difficult than it should be. But I have finally got my hands back on my organometallics text book so that I can continue in my history of organometallic chemistry. I last left off in 1917 with the synthesis of Lithium alkyls, which then brings our journey to 1919 with a German chemist named Franz Hein. This man's claim to fame came to him with the synthesis of polyphenylchromium compounds, being the first sandwich compounds to be made. This synthesis was performed by the mixture of CrCl3 (Chromium (III) Chloride) with PhMgBr (Phenylmagnesium bromide) to create a series of these phenylchromium salts that were later found to be made of biphenyl, not phenyl, and oriented in a way making them look like a sandwich.
Thursday, July 5, 2012
7/5/2012 - Synthesis of Thioridazine
Thioridazine is a drug currently used as a treatment for Schizophrenia and other psychotic ailments. The drug can also be used in the aid of people coming off of opiate withdrawal and insomnia. After the synthesis the drug exists in the racemic form (meaning two molecules mirror images to one another), which in some cases can cause a problem (See Thalidomide), but in this case both molecules are of bio-medical importance. The synthesis involves the combination of 2-methylthiophenothiazine with 2-(2-chloroethyl)-1-methylpiperidine through the alkylation of the nitrogen on the 2-methylthiophenothiazine by the addition of a weak base to deprotonate that nitrogen (in this case Sodium amide, NaNH2) so that a substitution reaction can occur between the Cl and the N.
Source: Wikipedia
Source: Wikipedia
Monday, July 2, 2012
7/2/2012 - Synthesis of Geosmin
The scent of rain is a very familliar odour for most, but most of those people have no clue why rain smells and question why rain should smell at all, it's just water. Well the smell doesn't come from the rain itself, but from the soil below it. The molecule in this scent is Geosmin, which also makes soil smell the way it does. It is synthesized by microbes in the ground and when the rain disturbs the soil, this molecule gets released into the air. Now chemists have found a way for us to make this molecule without the help of our little friends in the soil. It all begins with a simple condensation reaction with ethyl vinyl ketone, and 2-methyl-cyclohexanone in presence of sodium ethoxide (NaOEt) creating the second ring. But now there is an extra ketone on that second ring, so a second step of a special reduction method is required. This involves the addition of (CH2SH)2 with BF3 to create a thioketal derivative, and then desulfurization with Raney Nickel.
Source
Source 2
Source
Source 2
Labels:
chemistry,
condensation,
fact,
geosmin,
natural,
raney nickel,
reduction,
soil
Tuesday, June 12, 2012
6/12/2012 - Synthesis of Silver Iodide
One of my fellow chemists asked me if I knew where she could purchase a single silver iodide crystal, and after a while searching without an answer I looked up the synthesis of this metal compound. The procedure is fairly simple starting with dissolving silver nitrate (AgNO3) in water, followed with the addition of potassium iodide (KI). A yellow precipitate will form immediately containing both the α-AgI and the β-AgI crystals which can be separated through different dissolution methods.
AgNO3 (aq) + KI(aq) → KNO3 (aq) + AgI(s)
Source: O. Glemser, H. Saur "Silver Iodide" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 1036-7
Monday, June 11, 2012
6/11/2012 - Alkene to 1,2-Diol
Alkenes can be used in many sorts of organic reactions, and here is another use in the introduction of alcohol groups onto a molecule. A 1,2-diol is an organic molecule with two adjacent alcohol groups, as seen in the image below, and it is made through the reaction of an alkene with Osmium Tetroxide and a weak base, such as pyridine. Then there will be a workup of water and sodium bisulfite to protinate the alcohol groups. As seen below, highlighted in blue, the oxygen atoms come from the same osmium tetroxide molecule, resulting in the alcohol groups being syn to one another (being on the same side of the molecule).
Saturday, June 9, 2012
6/9/2012 - Alkene Halohydration
Sorry for the lack of posts recently as physiotherapy has taken over my life lately and it has exhausted me to the point of falling asleep super early (unlike me). But I thought I would continue in doing a couple basic organic reactions. This reaction is the conversion of an alkene into a halohydrated alkane, where there is both an alcohol group and a halogen added to the carbons. The reactants in this reaction are simply a diatomic halogen (F2, Cl2, Br2, I2) along with water which also works as the solvent. This results in the alcohol adding to the most substituted carbon with the halogen adding to the other carbon, both anti configuration to one another.

Saturday, May 19, 2012
5/19/2012 - Alkene to 1,2-dihalide
Labels:
1,
2-dihalide,
alkene,
chemistry,
dihalide,
organic,
organic chemistry
Saturday, May 12, 2012
Computer Programs for Chemistry Students
As an undergrad student you really don't have the money to spend on the professional drawing programs such as ChemDraw, or professional NMR processing programs like TopSpin, so what is out there for a starving undergrad to easily get through their Chemistry degree?
Chemistry Drawing:
Chemistry Drawing:
- ACD/ChemSketch (Windows): They provide both a paid version and a Freeware version for educational purposes, which I find as a very intelligent move on their behalf. This program is very easy to use and very capable in performing all the tasks required of an undergraduate program. It is the program I most frequently use to make Chemical illustrations.
- ChemDoodle (Mac + Windows + Linux): This company (iChemLabs) realized the need for an affordable Chemistry drawing program to make up for the ridiculous prices that are currently out there so they came out with ChemDoodle for a very reasonable $59.95 for single, and $89.95 for two copies. This company truly realizes the importance of quality chemical education so they research looking into the production of Chemistry web programs marked as Chemdoodle Web Components. On there they offer free solutions to add to your website through the use of HTML5, much like how Jmol uses Java (HTML5 being a much safer and stable option for your computer). Expect to see this technology coming up in ebook text books, chemistry blogs, and other educational places. Also if you have a smartphone they have a Chemdoodle app for you to work with while you aren't at home, or in the office. I strongly recommend this program to anyone in the chemical field.
NMR Programs:
- ACD/NMR Processor (Windows): Again this company offers a freeware version of there wonderful NMR processing software, which matches many paid solutions out there. This is the program I have been using for quite sometime now for interpreting NMR spectra.
- Chemdoodle (Mac + Windows + Linux): It also performs basic NMR functions, it isn't the greatest but it will get you along for what most undergrads are required to perform.
Publishing:
- Google Drive (Web): This has probably been the most used program for my undergraduate degree; I rely on it for all of my word processing and basic spreadsheets. When I need a more advanced spreadsheet program I have to use Excel, but these Google Docs programs are being improved very rapidly, so I suspect I may not need to rely on Microsoft Office for much longer. This makes life so much easier when you work on multiple computers such as using a public computer at school, or even borrowing someone else's computer to do some work on those lab reports that are due in an hour. And for group projects, it makes live collaboration possible, making the students' lives so much easier.
- Lyx (Mac + Windows): For those who know LaTeX, this is a very useful editor to make your documents look perfect. This is very useful for those of you who are Physical Chemists, Quantum Chemists, or anyone else who work heavily with mathematics. Or those Chemists who just really enjoy the styling of LaTeX documents.
- LibreOffice (Mac + Windows + Linux): Since windows has discontinued the release preview of their office 365, I've had to find an alternative. Now I don't need the full functionality of excel at this moment so I can settle for something else, but when that time comes I will have to invest in office again. This is a full functioning alternative to Office and I will recommend this open source project to anone.
Tuesday, May 8, 2012
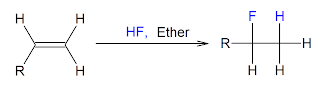
5/8/2012 - Alkene to Alkyl halide
There are many simple organic reactions out there in the world of chemistry and this here is one of them. This reactions involves the treatment of an alkene with a hydrogen halide and ether as the solvent. This results in the HX (HF, HCl, HBr, HI) to combine with the alkene agreeing with the Markovnikov rule (the halide adding to the most substituted carbon). The reaction can be seen below using HF as the hydrogen halide.
Thursday, May 3, 2012
What are Particles?
As I continued to read the biography on Paul Dirac, The Strangest Man by Graham Farmelo, I came across this very ensightful quote of Dirac's which gives a very good sense on how we can imagine particles.
When you ask what are electrons and protons I ought to answer that this question is not a profitable one to ask and does not really have a meaning. The important thing about electrons and protons is not what they are but how they behave - how they move. I can describe the situation by comparing it to the game of chess. In chess, we have various chessmen, kings, knights, pawns and so on. If you ask what a chessman is, the answer would be [that] it is a piece of wood, or a piece of ivory, or perhaps just a sign written on paper, [or anything whatever]. It does not matter. Each chessman has a charateristic way of moving and this is all that matters about it. The whole game of chess follows from this way of moving the various chessmen.Source: Farmelo, Graham. The Strangest Man. Basic books, Philidelphia, 2009.
Sunday, April 29, 2012
4/29/2012 - Synthesis of Lithium Alkyls
As I swerve back into the history of Organometallics I want to start back to 1917 in Germany. A chemist named Wilhelm Schlenk, at the University of Munich, determined how to synthesize organolithium compounds through a Transalkylation process coupled with organomercury compounds. He came up with two different methods of synthesis, one where there is nothing attached to the Lithium in the beginning and then the other method where there is an organic group that is transferred to the Mercury with a shorter alkyl group. Both reactions are seen here below.
Tuesday, April 24, 2012
The Immortality of Science
Here is a quote by Max Planck, touching on the subject of important scientific breakthroughs:
A scientific truth does not triumph by convincing its opponents and making them see the light, but rather because its opponents eventually die and a new generation grows up that is familiar with it.
Friday, April 20, 2012
To Describe is to Undescribe
Quantum mechanics is a peculiar subject to describe to someone who has very little knowledge of mathematics since most of the contents of the subject involve 'things' that have no visual analogies to the classical physics world we live in. This topic has been orbiting around my mind ever since being introduced to the obscure topic, which told me that these particles can only be represented and understood through the mathematics that place foundation for theory. For me as a student, whom had nothing more than the required mathematical background, it was difficult to pry myself away from my haunting visual of particles to grow solely dependent on the mathematics. This modern physics was the first to venture off into a realm of indescribability, where the physics could not be allegorical to anything built in our sensory system. I am currently reading a biography on the quantum physicist Paul Dirac, who being a very quiet person already, only saying the most necessary words as if it was costly to say anything more than what was required, stated this very beautiful quote below, when his mother asked him to explain to her what he works on. The beauty of this quote much relates to the philosophies described in Taoism, where all meaning is lost if one attempts to describe it through words.
“To draw its picture is like a blind man sensing a snowflake. One touch and it’s gone.”
Wednesday, April 18, 2012
4/18/2012 - How the Atomic Makeup of Water was Discovered
The question of how did someone could find out what is in water is a simple one, it basically consisted of combining hydrogen and oxygen together in a combustion to yield out water. The scientist who carried out these experiments first was none other than Henry Cavendish, the British man to have the Cavendish Laboratory in Cambridge to be named after, of which many famous physicists go through, including Paul Dirac, James Maxwell, and Ernest Rutherford. Now at that time, he had not named these gases as Hydrogen and Oxygen, but rather dephlogisiticated air and phlogiston respectively. Phlogiston being the fire element that is released during combustion. Through many experiments he determined that the greatest efficiency of water was obtained through the use of two parts hydrogen gas with one part of oxygen gas. This was a very skeptical idea at the time that water was produced out of two invisible gases, but he endured many more of his experiments to prove his hypothesis, and win respect of the skeptics.
2H2 + O2 → 2H2O + Heat
Source: Jaffe, Bernard. Crucibles: The Story of Chemistry. London, Hutchinson's scientific and technical publications, 1931.
Tuesday, April 17, 2012
4/17/2012 - The First Synthesis of Ammonium Chloride
The first recorded synthesis of this ammonium chloride compound was performed by Joseph Priestly, the minister responsible for my last post. This was done while he was again experimenting with various gasses, and this time he was working with ammonia, seeing what would happen if he collecting a gas over mercury given off by ammonia water. This gas was colorless, but contained the most pungent odor, so he took it even further by bringing the ammonia gas together with the hydrogen chloride gas. This reaction produced a cloud, which after a while settled to form an odorless white powder. He came to terms that this was something new when he analyzed it and noticed that there was no pungent odor from either the ammonia, nor the HCl.
NH3(aq) + HCl(aq) → NH4Cl(aq)
Thursday, April 12, 2012
4/12/2012 - Creation of Soda Water
In the late 18th century a Chemist by the name of Joseph Priestly, an self educated man living in Birmingham at the time, had an everlasting love for science and chemistry, so he decided to pursue science over his current ministerial work. He was strong on the experimental method and had his scientific love focused on gases and the sources of the gas. One day when he was at a public brewery he noticed the bubbles coming out of the vat and thought to himself what this gas was, so he decided to see what would happen if he would put a flame near these gases and observed that the flame extinguished after exposure. So he collected this gas, and figured out a way to make it at home to analyze it, and little did he know that this gas was in fact carbon dioxide, CO2. In one experiment he bubbled it through water and noticed that it was slightly soluble, and the water turned into an exceedingly pleasant sparkling water, very much like Seltzer water. This was brought to the attention of the Royal Society and later became known as Soda water
Tuesday, April 10, 2012
4/10/2012 - Synthesis of Ethylene
A very simple organic compound named ethylene, C2H4 was first synthesized by the ancient alchemist named Johann Becher. This compound of two carbons, containing a double bond was first synthesized by a process called ethanol dehydration where absolute ethanol is passed over a concentrated solution of sulfuric acid in the reaction C2H5OH + H2SO4 → C2H4 + H2O +HSO4. This compound later was introduced in 1922 as an anesthetic by Dr. Lockhardt, and is used today for hundreds of other purposes.
Monday, April 9, 2012
Beginnings of Chemistry: Paracelsus
This post is a divergence from my usual reaction of the day, and more of a history lesson on one of the founders of what science is today, named Paracelsus. Paracelsus was born in 1493 into an age of alchemy, where most men of that science were striving to turn anything into the valuable element of gold. So naturally, as a man in love with the natural world, he would be immersed into the world of science (alchemy, medicine, astronomy, and botany). In a lifetime of chasing this witchcraft and non scientific method of medicine, he finally had his epiphany in seeing that there were better methods in the science of medicine. He found that it was better to search for drugs, to then prepare and purify them for use, than to use the previous methods of healing. An example of this was that he was the first to make a tincture of opium, which was named by him laudanum, where he determined that the opium alkaloids were more soluble in alcohol than water and resulted in him making this laudanum solution. At first people were skeptical of him because of his title as alchemist, meaning that he was related to the history of alchemists failing at producing gold. Because of this he found it his mission to change peoples opinions of alchemy and science. He gives a beautiful quote, that has much relevance still today for science skeptics:
Source:
Jaffe, Bernard. Crucibles: The Story of Chemistry. Hutchinson's Scientific and Technical Publications, London, 1931
"It's name will no doubt prevent its being acceptable to many; but why should wise people hate without cause that which some others wantonly misuse? Why hate blue because some clumsy painter uses it? Which would Caesar order to be crucified, the thief or the thing he had stolen? No science can be deservedly held in contempt by one who knows nothing about it. Because you are ignorant of alchemy you are ignorant of the mysteries of nature."In changing the mission of the 16 century alchemist, he also set out to change the methods of the physician stating clearly that "if the physician were not skilled to the highest degree in alchemy, all his art was in vain". With today's sciences we see clearly that he set future scientists in the right direction, and produce technology to better the human race.
Source:
Jaffe, Bernard. Crucibles: The Story of Chemistry. Hutchinson's Scientific and Technical Publications, London, 1931
Labels:
15th century,
alchemy,
chemistry,
history,
laudanum,
medicine,
opium,
paracelsus
Friday, April 6, 2012
Happy Easter From Chemistry
Here is a fun chemistry video on the contents of a creme egg. There is nothing better than a holiday themed chemistry experiment.
Wednesday, April 4, 2012
4/4/2012 - What Makes Old Paper Yellow?
I apologize for once again another prolonged absence, for I have started physiotherapy and the pain has risen significantly, and writing a blog post was the least of my concerns. I don't currently have my organometallic text book on me at this moment, so I thought I would diverge from the organometallic history for a moment to talk about paper.
If you have ever studied upon an old piece of paper, you would have noticed that the paper is no longer white but appears yellow. If you were me, you would have had risen two questions, one, whether the paper was made in this tone, or two, whether some chemical reaction had been performed on the paper to make it this colour. So I decided to look at what actually happens to the paper and have found that what makes it yellow is the formation of the molecules called Chromophores. This process is done through the oxidation of cellulose over time creating many different products, but of most importance the aldehydic chromophores responsible for the yellowing.
Finding this information receives it's importance when researchers want to look into restoring old papers through a reduction process removing these chromophores.
Source: Science Magazine
If you have ever studied upon an old piece of paper, you would have noticed that the paper is no longer white but appears yellow. If you were me, you would have had risen two questions, one, whether the paper was made in this tone, or two, whether some chemical reaction had been performed on the paper to make it this colour. So I decided to look at what actually happens to the paper and have found that what makes it yellow is the formation of the molecules called Chromophores. This process is done through the oxidation of cellulose over time creating many different products, but of most importance the aldehydic chromophores responsible for the yellowing.
Finding this information receives it's importance when researchers want to look into restoring old papers through a reduction process removing these chromophores.
Source: Science Magazine
Monday, March 26, 2012
3/26/2012 - Synthesis of Arsphenamine
At this point in time, in 1909, medicine was very limited on what cured versus what merely treating a disease, and there were many breakthroughs in this field all of the time. A year prior to this date a man named Paul Ehrlich invented the chemotherapy method in fighting cancer through using toxic compounds. With his knowledge of toxic material he was experimenting on what other diseases could be helped through this method and he synthesized a molecule called Arsphenamine, or also known as Salvarsan, used to treat syphilis. It was found through synthesizing hundreds of organic arsenical compounds and testing each one. This was also the first organic antisyphilic, meaning that before this compound was produced, syphilis was only treated with inorganic compounds such as Mercury.
Tuesday, March 20, 2012
3/20/2012 - Synthesis of Iodotrimethyl Platinum (IV)
An english chemist of the name William J. Pope worked in researching stereochemistry, but one of his major achievements, in 1909, came with the synthesis of IodoTrimethyl Platinum, or (CH3)3PtI. This synthesis was carried out through the mixing of Potassium chloroplatinate, K2PtCl6, along with a Grignard methyl complex, CH3MgI. This compound holding it's historical significance through the fact that it became the first σ-organotransition-metal compound meaning that the organic group is connected through a sigma bond to the transition metal.
Sunday, March 18, 2012
3/18/2012 - Synthesis of Diphenylsilicone
At the turn of the century in 1901, there is a chemist named L. Kipping working strongly with different silicone chemistry. This year he synthesizes a molecule he calls to be diphenylsilicone despite stating of a much higher molecularity.
Saturday, March 17, 2012
3/17/2012 - Barbier and the Grignard Reactions
And once again I apologize for my prolonged absence from the blog as with my injury it induces pain that lasts for a good period of time, and then it lets off for a couple days, only to drive right back into the pain. Some of these times, I really can't find it in me to do a blog post even though I have so much free time condemned to me.
As the organometallic history progressed, in 1899, a man by the name of P. Barbier investigated reactions involving an alkyl halide being used as an addition reaction onto strongly electrophilic points. These reactions were usually involving using reactants such as: an alkyl halide, Zinc, and followed by an aqueous work up. He began studies focused on finding other metals that could have similar use in these kinds of reactions. He concluded that adding Magnesium instead of Zinc consequented in a more efficient addition. His student Grignard followed up by effectively describing the reaction in more detail resulting in the reaction being named after him and also with winning the Nobel Prize.
will turn into
As the organometallic history progressed, in 1899, a man by the name of P. Barbier investigated reactions involving an alkyl halide being used as an addition reaction onto strongly electrophilic points. These reactions were usually involving using reactants such as: an alkyl halide, Zinc, and followed by an aqueous work up. He began studies focused on finding other metals that could have similar use in these kinds of reactions. He concluded that adding Magnesium instead of Zinc consequented in a more efficient addition. His student Grignard followed up by effectively describing the reaction in more detail resulting in the reaction being named after him and also with winning the Nobel Prize.
will turn into

Tuesday, March 13, 2012
3/13/2012 - Synthesis of Nickel tetracarbonyl
About a decade after Mendeleev made his predictions of Eka-Si(C2H5)4, in 1890, a scientist by the name of Ludwig Mond became the first to synthesize Nickel tetracarbonyl, Ni(CO)4. This was performed by passing carbon monoxide over Nickel metal to make a light yellow liquid. This technology was then harnessed to refine nickel when Mond founded the company Imperial Chemical Industries.
 |
| Nickel Tetracarbonyl |
Monday, March 12, 2012
3/12/2012 - Synthesis and Predicted Properties of Tetraethylgermanium
Sorry for my week long absence, I was quite busy with my girlfriend visiting me during her spring break, and combined with my injury, I didn't feel up to writing in my blogs. I did, however, start playing around with a Wordpress blog that I just created. I am loving their integration with LaTeX in the blog capabilities, and right now I am wishing that I had gone with Wordpress instead of Blogger. The blog I started is just a blog where I intend to record down what I have been learning in my studies in order to help me in my memory of the subjects.
As for the continuation of the organometallic history, in 1971 Dmitri Ivanovich Mendeleev set off to organize the periodic table in according to their physical properties (both atomic weight and valence). In this certain test where he knew the properties for Si(C2H5)4 and Sn(C2H5)4, and knew that there was an element below Silicon and above Tin, he could predict the properties of the organometallic of Ekasilicon Eka-Si(C2H5)4 (later named Ge(C2H5)4). The properties predicted were the density and the boiling point, and the predictions were only 0.03 points below the actual density and 3.5 degrees C below the actual boiling point.
As for the continuation of the organometallic history, in 1971 Dmitri Ivanovich Mendeleev set off to organize the periodic table in according to their physical properties (both atomic weight and valence). In this certain test where he knew the properties for Si(C2H5)4 and Sn(C2H5)4, and knew that there was an element below Silicon and above Tin, he could predict the properties of the organometallic of Ekasilicon Eka-Si(C2H5)4 (later named Ge(C2H5)4). The properties predicted were the density and the boiling point, and the predictions were only 0.03 points below the actual density and 3.5 degrees C below the actual boiling point.
 |
| Tetraethylgermanium |
Friday, March 2, 2012
The Grounding Problem of Human Consumption
The highest issue regarding how humans consume goods (take that as electronics, food, etc.) is that the wasted goods don't follow a pattern that exists in most biological and chemical systems: That being a cycle. Life has harnessed the use of chemical cycles ever since there was life in the idea that a cycle exists as an efficient form, to reuse or remake from what has already been produced and used. The point of a cycle is to expend the minimum amount of energy, and in the chemical world, this energy exists with chemical bonds. For a chemical system, for example being that of the ATP cycle (adenosine triphosphate) our body breaks this down into ADP harnessing the energy of the phosphate-phosphate bond to power other chemical processis. Now after the ADP is produced, the cell does not just toss this out of the cell into a landfill, but rather remakes ATP by adding another phosphate through the actions of oxidative phosphorylation (or by other biochemical systems) producing a cyclic existence of ADP and ATP. If the cell were to throw out the ADP, the process would be extremely inefficient where it would have to use much for energy to produce more chemical bonds to synthesize a whole molecule of ATP.
For starters this issue can be resolved greatly by our recycling system, but still manufactures still tend to use non recyclable plastics in many packagings. Just last week I purchased a bag of candy, and it had a large non-recyclable packaging, but even more astonishing, I opened up the package to find that every miniature candy was unnecessarily individually packaged, in again, non-recyclable plastics. This consumerism is always so evident during boxing day shopping, or anything of the sort, when people purchase just because it's on sale, where they have conquered the price, but really, have no need for the product. I volunteer with a pre-teen youth group, and in hearing when they brag about what their parents buy them, I wonder how long it will take until those parents just toss those items to make room for more. To overcome this hurdle, one must donate those toys to the less fortunate, something that many of the more fortunate parents don't do because they dont realize the importance. A chemical analogy to this would be a cell in an ATP rich environment, where it would more rather use the ATP in the environment than to remake ATP out of the used up ADP. This cell would adapt, and may have less mitochondrial systems to make its own ATP, just as the parents create this non-cyclic system of consuming because they can take in products from the outside environment so easily.
This idea also exists in our clothing. Back when it was harder to get new clothes, people used to mend and edit their clothes themselves instead of throwing something away at the sign of degradation, keeping the clothing in a cycle. And currently the quality of today's clothing is appalling, manufacturers produce these clothes in the philosophy that the consumers will buy more if they go through their products faster. And if the consumers do get angry at the quality, the manufacturers wont necessarily have to deal with it. If this was back in the day when consumers purchased local made clothing, if the consumer found something wrong, they could take it up with the tailor himself, resulting in the tailor wishing to produce a well made product.
One of the biggest solution of this would be for governments to help out local companies so that it would be possible to compete with Chinese products sold in stores like Walmart and other enormous, money eating stores. My parents used to own a display fixtures company produced out of wood, and this company went down for this very reason. Our customers ended up buying cheaply made fixtures from Chinese manufacturers, instead of purchasing our quality products. I can still go into stores today, and see items we had made about ten years ago, where the cheaper Chinese products would get tossed very quickly.
Discovery after discovery in the chemical world reveals endless amounts of cycles evident in our universe. It is only common sense to infer that within a cyclic system, the laws of thermodynamics are most favorable. Once this realisation sets in, one will see anything that doesn't adhere to this system and understand that a non-cyclic system is doomed for failure in the long run. And for the cell in the ATP rich environment, once there is no more ATP supplied to it, it won't know what to do to produce any ATP for itself.
For starters this issue can be resolved greatly by our recycling system, but still manufactures still tend to use non recyclable plastics in many packagings. Just last week I purchased a bag of candy, and it had a large non-recyclable packaging, but even more astonishing, I opened up the package to find that every miniature candy was unnecessarily individually packaged, in again, non-recyclable plastics. This consumerism is always so evident during boxing day shopping, or anything of the sort, when people purchase just because it's on sale, where they have conquered the price, but really, have no need for the product. I volunteer with a pre-teen youth group, and in hearing when they brag about what their parents buy them, I wonder how long it will take until those parents just toss those items to make room for more. To overcome this hurdle, one must donate those toys to the less fortunate, something that many of the more fortunate parents don't do because they dont realize the importance. A chemical analogy to this would be a cell in an ATP rich environment, where it would more rather use the ATP in the environment than to remake ATP out of the used up ADP. This cell would adapt, and may have less mitochondrial systems to make its own ATP, just as the parents create this non-cyclic system of consuming because they can take in products from the outside environment so easily.
This idea also exists in our clothing. Back when it was harder to get new clothes, people used to mend and edit their clothes themselves instead of throwing something away at the sign of degradation, keeping the clothing in a cycle. And currently the quality of today's clothing is appalling, manufacturers produce these clothes in the philosophy that the consumers will buy more if they go through their products faster. And if the consumers do get angry at the quality, the manufacturers wont necessarily have to deal with it. If this was back in the day when consumers purchased local made clothing, if the consumer found something wrong, they could take it up with the tailor himself, resulting in the tailor wishing to produce a well made product.
One of the biggest solution of this would be for governments to help out local companies so that it would be possible to compete with Chinese products sold in stores like Walmart and other enormous, money eating stores. My parents used to own a display fixtures company produced out of wood, and this company went down for this very reason. Our customers ended up buying cheaply made fixtures from Chinese manufacturers, instead of purchasing our quality products. I can still go into stores today, and see items we had made about ten years ago, where the cheaper Chinese products would get tossed very quickly.
Discovery after discovery in the chemical world reveals endless amounts of cycles evident in our universe. It is only common sense to infer that within a cyclic system, the laws of thermodynamics are most favorable. Once this realisation sets in, one will see anything that doesn't adhere to this system and understand that a non-cyclic system is doomed for failure in the long run. And for the cell in the ATP rich environment, once there is no more ATP supplied to it, it won't know what to do to produce any ATP for itself.
Thursday, March 1, 2012
3/1/2012 - Synthesis of the First Metal Carbonyl Complex
In 1868 a French man by Marcel-Paul Schützenberger (a mathematician who also studied medicine) began a new form of organic complexes by creating the first metal carbonyl complex. These complexes gain importance through the catalytic reactions of Carbon monoxide, which is a piece in the creation of many molecules of biological importance. The bonding of the CO with the metal is also of some relevance due to the unique character (not shown in the picture below) where the orbitals of the metal bond with the pi-orbitals of the C≡O. The complex, Dicarbonyldichloroplatinum [Pt(CO)2Cl2] was formed through him passing carbon monoxide and chlorine over powdered Platinum (also known as Platinum black).
Wednesday, February 29, 2012
2/29/2012 - Synthesis of Diethylmagnesium
Up to the point in 1866 there had been no way of synthesizing a magnesium organometallic compound without having attached a halide to the complex. James Alfred Wanklyn (famous for methods of determining water quality) came up with a method of synthesis to exclude any presence of a halide anywhere in the synthesis. This involved the use of Frankland's development of diethylmercury and mixing it in dissolved magnesium yielding a mixture of diethylmagnesium and dissolved mercury.
Tuesday, February 28, 2012
2/28/2012 - Synthesis of Organochlorosilanes
Again following the history of organometallics, a very important duo comes into the scene Charles Friedel, and James Mason Crafts (the brains behind the technology of Friedel-Crafts reactions) and harnessed their chemistry to the production of organochlorosilanes. These molecules create the very important bond between a Carbon and a Silicon atom in many different mole ratios (up to 4 different Carbon-Silicon bonds around the silicon center). The way this reaction is performed is through the addition between a silicon tetrachloride and a alkylzinc compound to produce the organochlorosilane and a zinc chloride salt.
Monday, February 27, 2012
2/27/2012- Synthesis of Alkylaluminum Iodides
Next in the realm of organometallic chemistry, taking place in 1859, was the creation of alkylaluminum iodides. The scientists involved in the invention of this technology were Wilhelm Hallwachs (discovery of the photoelectric effect), and A. Schafarik, when they took a mixture of dissolved aluminum and added a solution of alkyliodides (any primary R group). This reaction produced a mixture of di-addition alkylaluminum iodide and mono-addition alkylaluminum iodide.
Sunday, February 26, 2012
2/26/2011 - Synthesis of Tetraethyllead
Again, I apologize for the delay in posting due to my ailments, but I shall remain posting in the theme of organometallic history. Just as a disclaimer, most of the information for these posts I have been gathering from Elshenbroich and Salzer's Organometallics 2nd Eddition. Following this chronology brings us to two other chemists studying in 1852, Carl Jacob Löwig (discoverer of the element bromine) and Eduard Schweizer. While studying lead organometallics in Zürich, they became the first to prepare tetraethyllead by using ethyliodide and an allow of Sodium and Lead (much like the Frankland synthesis).
4NaPb + 4(C2H5) → (C2H5)4Pb + 4Na + 3Pb
Following this synthesis they continued to produce (C2H5)3Sb and (C2H5)3BiTuesday, February 21, 2012
UBC Chemistry: Recommended Changes
After completing (well on the verge of completion) I would like to note a couple of my suggestions of which pertain to the changes they should make in the program. First off I would like to mention that there are no computer science credits required to complete this degree, and in my opinion this is wrong. For the physical sciences of physics and chemistry, one of the growing fields in these two topics is the computational aspect. Chemistry is no longer just experiments in the chemical world, but it also ventures into the tools of computing. At this point in my university career I regret not having taken one as I try with much difficulty to teach myself coding languages in the attempts to learn more computational skills. Also within the major program (already contained in the honours program) Chem 320: Structures of Atoms and Molecules demands to be a required course as it intros computational methods in chemistry. The chemistry department does recognize the changing field, but it must look into enforcing these changes onto the students.
Another change of which is needed is a history of chemistry course. Yes, this sounds redundant to learning the aspects of chemistry, but in my education I found myself bottling up all this theoretical knowledge without knowing how this theoretical knowledge was produced. I have talked to a number of my graduating classmates and most of them agree that we have little knowledge of how the scientific method works. So if the chemistry department was to enforce students to take a history course of chemical experiments I believe we will be more prepared to enter the world as scientists instead of entering the world as hardened students. This idea came to me as one of my organic professors, who always gave small history lectures amidst the curricular lectures, taught me how this organic technology came to exist; with this knowledge I grasped a better understanding of what it was that creates a great discovery.
This isn't a complete posting, so as I think of additional issues with the Chemistry program at UBC I will add them in here. And in the next couple of days I will include a suggestions post for anyone starting out in the UBC chemistry program.
Another change of which is needed is a history of chemistry course. Yes, this sounds redundant to learning the aspects of chemistry, but in my education I found myself bottling up all this theoretical knowledge without knowing how this theoretical knowledge was produced. I have talked to a number of my graduating classmates and most of them agree that we have little knowledge of how the scientific method works. So if the chemistry department was to enforce students to take a history course of chemical experiments I believe we will be more prepared to enter the world as scientists instead of entering the world as hardened students. This idea came to me as one of my organic professors, who always gave small history lectures amidst the curricular lectures, taught me how this organic technology came to exist; with this knowledge I grasped a better understanding of what it was that creates a great discovery.
This isn't a complete posting, so as I think of additional issues with the Chemistry program at UBC I will add them in here. And in the next couple of days I will include a suggestions post for anyone starting out in the UBC chemistry program.
Saturday, February 18, 2012
2/18/2012 - Synthesis of Dimethylmercury
In the following years Edward Frankland continued researching organometallic compounds as he realized how much new chemistry can be harnessed from these studies. In 1852 he branched into Mercury chemistry and then following into Tin and Boron reactions. With these reactions he focused on simple methyl additions onto the metal creating a linear dimethylmercury through the reaction 2CH3X + 2Na + Hg → (CH3)2Hg + 2NaX. This dimethylmercury compound is extremely toxic, to the degree that one drop on a latex glove will absorb through and absorbs into the skin and months later the fatality takes over. And as stated before he transcended these alkylhalide reactions into adding different R groups onto Hg, Sn, and B.
Friday, February 17, 2012
2/17/2012 - Preparation of Ethyl Radical
Bunsen took on students at Marburg, and one of those students was Edward Frankland and he set out to synthesis an ethyl radical from the combination of 3C2H5I and 3Zn, thinking that would create ZnI2 and 2C2H5, but instead revealed that the products were a pyrophoric liquid of (C2H5)2Zn (diethylzinc), ZnI2, and a solid of C2H5ZnI. He also harnessed this technology to make the methyl analogue to synthesize dimethylzinc as well. I do realize that I have already done a post regarding the synthesis of diethylzinc, but this here was the historical method of the first sythensis, different from todays methods. Sorry again for my absences, I have been under a significant amount of pain.
Saturday, February 11, 2012
2/11/2012 - Studies of Alkarsine Derivatives
Then in 1840 Robert Bunsen continued the studies of the arsenic compounds synthesised in Paris a century earlier (cacodyl compounds). He used these compounds to synthesize a numbed of derivatives of R2As-AsR2 into molecules (CH3)2AsCN. After the synthesis Bunsen supposedly tasted this dangerous concoction, and luckily he lived to tell the tale.
Photo source
Photo source
Friday, February 10, 2012
2/10/2012 - Synthesis of Zeise's Salt
Thursday, February 9, 2012
2/9/2012 - Cacidyl oxide synthesis
As an apology for my absence my next couple of reactions will follow along the chronological order for the history of organometallic chemistry. The first ever synthesis of an organometallic occured in Paris, in 1760, at a military pharmacy, where there was a cadet who was working in creating synthetic inks containing cobalt salts, and in extracting the cobalt from the raw form arsenic was removed. In this removal the As2O2 is combined with CH3COOK to produce a fuming liquid containing the organometallic product of [(CH3)2As]2O (also known as Cacidyl oxide)
Thursday, February 2, 2012
2/2/2012 - Stille Reaction
This organometallic catalyzed reaction causes the creation of a carbon carbon bond between an organotin compound (R1SnBu3) and an sp2 hybridized organohalide (R2-X). The reaction can be thought of as in a reaction cycle to better visualize the reactions going on here. The catalyzing material here is the Paladium complex with a number of variable ligands surrounding it. This reaction was discovered by the chemist John Kenneth Stille in 1977 and is continued to be used in many organic synthesis processes used for molecules of biomedical significance.
Wednesday, February 1, 2012
Methane Storage
Here is a wonderful video that I found while looking through a number of science educational videos. Something that I've always found interesting is the creation of educational videos, and I think this one is wonderful.
Sunday, January 29, 2012
1/29/2012 - Pharaoh's Serpent
This reaction doesn't involve the mixing of chemicals, but instead an exothermic reaction when the compound is in contact with a significant enough heat source. The compound needed for this reaction is Mercury (II) thiocyanate or Hg(SCN)2, which is an incredibly toxic compound, so I advise you not to try this reaction at home, due to the toxic gases and the toxicity of the compound. The reaction was discovered by Wohler in 1821 right after his first synthesis when he observed the winding, worm-like projections.
Thursday, January 26, 2012
1/26/2012 - Synthesis of Imines from Alcohols Catalyzed by a Ruthenium Complex
This reaction involves turning a primary alcohol into an imine product with the alleviation of hydrogen gas, catalyzed in the presence of a Ruthenium N-Heterocyclic Carbene complex in the presence of DABCO (which acts as a ligand). The mechanism is quite complicated but it incolves the exchange of Cl's for H's onto the Ruthenium, and then the addition of the alcohol, followed by the loss of H2, and then the addition of the amine, and then the alleviation of the final imine product. This reaction was researched by Agnese Maggi and Robert Madsen out of the Department of Chemistry at the Technical University of Denmark.
Picture: from source.
Source: Organometallics 2012, 31, 451−455
Wednesday, January 25, 2012
1/25/2012 - Activation and Functionalization of a Linear Alkane through Tungsten Complex
Here is another reaction involving the activation of a carbon hydrogen bond where after the activation the alkane can be functionalized through the addition of iodine into the system under liquid nitrogen. The study was performed out of my home department of chemistry at the University of British Columbia done by Peter Legzdins, Jenkins Y.K. Tsang, and Miriam S.A. Buschhaus in 2007. The tungsten complex was particularly a tungsten allyl nitrosyl complex, which allows the linear ƞ3 alkane to be attacked by the I2 in creating the selective alkyl halide at the terminal position (1-iodopentane here specifically).
Image: From source
Source: J. AM. CHEM. SOC. 2007, 129, 5372-5373
Image: From source
Source: J. AM. CHEM. SOC. 2007, 129, 5372-5373
Tuesday, January 24, 2012
1/24/2012 - Oxidative Addition of the Carbon-Hydrogen Bonds of Neopentane to a Iridium(I) Complex
In this organometallic reaction we are presented with a carbon hydrogen bond activation where the bond is cleaved. These reactions are very important in modern chemistry as it is able to turn very cheap organic molecules into functionally expensive organic molecules. In this reaction in particular, developed in 1982 by J.K Hoyano and W.A.G Graham out of the University of Alberta, it harnesses photochemical energy to cause an Iridium (I) organometallic complex to take up the neopentane molecule and to strip a hydrogen from the alkane. The resulting complex contains the same number of metal carbon bonds, as there is a CO group leaving during the reaction, but there is a new Metal hydrogen bond created as the activation.
Image: Taken from source below
Source: J.K Hoyano, W.A.G Graham. J. Am. Chem. SOC. 1982, 104, 3123-3125
Image: Taken from source below
Source: J.K Hoyano, W.A.G Graham. J. Am. Chem. SOC. 1982, 104, 3123-3125
Monday, January 23, 2012
1/23/2012 - Biological Organometllics: Zinc protoporphyrin
When asking someone of an example of an organometallic molecule within a human, one would usually answer with Hemoglobin, but there also exsists another within the red blood cells. Zinc protoporphyrin is produced by the red blood cells under the conditions of lead inhibition of hemoglobin, or merely the lack of iron to produce the hemoglobin. Because of this the presence of this molecule can act as a diagnostic tool to identify lead poisoning and a numerous number of ailments involving the red blood cells. And when looking at the chemical structure below, it can be observed that with a simple replacement of an Iron, it can act as an oxygen carrier as there is a heme group in it.
Sunday, January 22, 2012
1/22/2012 - Synthesis of Diethylzinc
I do apologize for my lengthy absence for I had a medical complication that has unfortunately also put my education on hold, but I will promise myself to not lose my chemical knowledge due to this lengthy vacancy, so I think it is best for me to keep up this blog regularly to take my mind off the pain. As for my first reaction back I have decided to give one that involves an organometallic (that being my passion).` This just being a Zinc compound arranged in a linear fashion connected to two ethyl molecules creating a Diethylzinc complex. I chose this to chow the simplicity in the synthesis of these complexes even though it exists in highly complicated bonding. The reaction was first accomplished in 1848, and later improved to using a 1:1 mixture of ethyl bromide and ethyl iodide (both sources of an ethyl+) and an active Zinc compound such as an alloy of Zinc and Copper.
Subscribe to:
Comments (Atom)